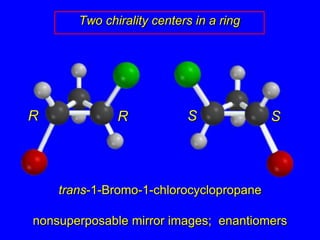
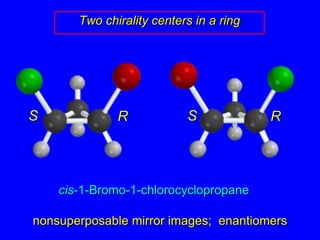
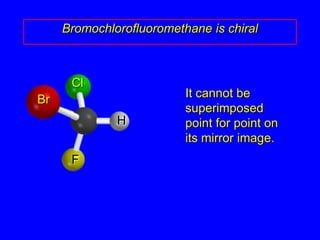
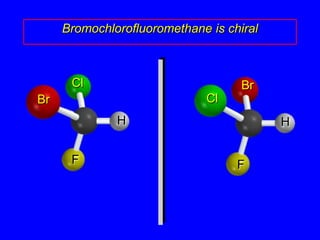
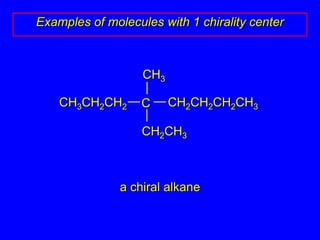
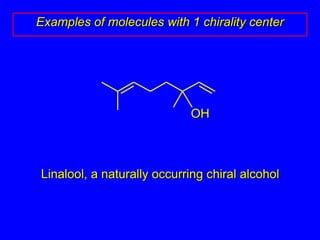
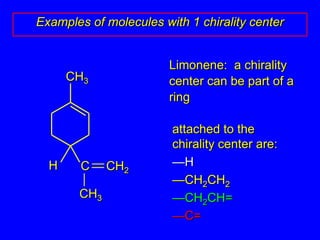
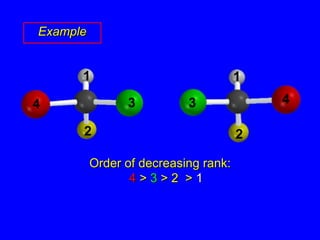
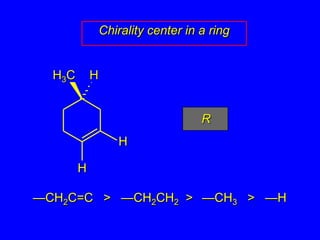
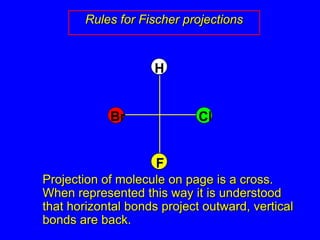
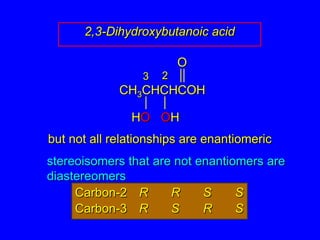
The document discusses different types of isomers including constitutional isomers, stereoisomers, enantiomers, and diastereomers. It explains chirality and how molecules can be chiral if they are non-superimposable on their mirror images. The Cahn-Ingold-Prelog system for assigning R/S configurations is described, which involves ranking substituents and determining clockwise or counterclockwise orientation. Enantiomers have opposite optical rotations while diastereomers can have any optical rotation. Molecules can have multiple chiral centers leading to multiple stereoisomers that may be enantiomers or diastereomers.


























![CH3CHCH2CH3CH3CHCHCH2OHOHRelative configurationPd[] + 33.2°[] + 13.5°No bonds are made or broken at the chirality centerin this experiment. Therefore, when (+)-3-buten-2-ol and (+)-2-butanol have the same sign of rotation, the arrangement of atoms in space is analogous. The twohave the same relative configuration.](https://image.slidesharecdn.com/estereoq-111016194322-phpapp01/85/Estereoq-33-320.jpg)
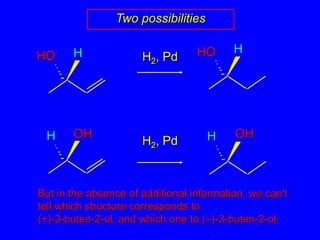
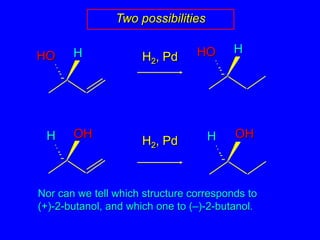
![HHOHHOOHOHHHAbsolute configurationsH2, Pd[] +33.2°[] +13.5°H2, Pd[] –13.5°[] –33.2°](https://image.slidesharecdn.com/estereoq-111016194322-phpapp01/85/Estereoq-36-320.jpg)
![CH3CH2CHCH2BrCH3CH2CHCH2OHCH3CH3Relative configurationHBr[] -5.8°[] + 4.0°Not all compounds that have the same relativeconfiguration have the same sign of rotation. No bondsare made or broken at the chirality center in thereaction shown, so the relative positions of the atoms are the same. Yet the sign of rotation changes.](https://image.slidesharecdn.com/estereoq-111016194322-phpapp01/85/Estereoq-37-320.jpg)



















![CO2HCO2H[] = -9.5°[] = +9.5°RSHOOHHHenantiomersOHHOHHRSCH3CH3CO2HCO2HSROHHOHHenantiomersOHHHOHRS[] = -17.8°[] = +17.8°CH3CH3](https://image.slidesharecdn.com/estereoq-111016194322-phpapp01/85/Estereoq-60-320.jpg)
![CO2HCO2HRSHOOHHHOHHOHHRSCH3CH3diastereomersCO2HCO2HSROHHOHHOHHHOHRSCH3CH3[] = -9.5°[] = +9.5°enantiomersenantiomers[] = -17.8°[] = +17.8°](https://image.slidesharecdn.com/estereoq-111016194322-phpapp01/85/Estereoq-63-320.jpg)