Lec11.pdf
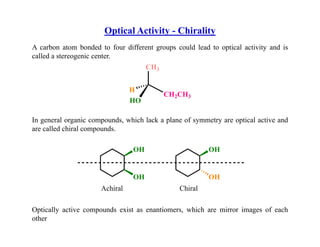
- 1. Optical Activity - Chirality A b t b d d t f diff t ld l d t ti l ti it d i A carbon atom bonded to four different groups could lead to optical activity and is called a stereogenic center. CH3 CH2CH3 HO H In general organic compounds, which lack a plane of symmetry are optical active and are called chiral compounds. OH OH OH OH Achiral Chiral Optically active compounds exist as enantiomers, which are mirror images of each other
- 2. Optical Activity - Chirality cis-1,2-dichlorocyclohexane If enantiomers are in equilibrium with each other through ring flipping, one enantiomer q g g pp g, cannot be separated from the other. Cl Cl Cl Cl Cl Cl Cl Cl Cl Ring flip 120°
- 3. Optical Activity - Chirality Enantiomers trans-1,2-dichlorocyclohexane Cl Cl Cl Cl Cl Cl Ring flip Cl Cl Cl Cl
- 4. Absolute Configuration: Cahn-Inglod-Prelog Rule Atom or Reason for Priority: First Point of Difference Substituents on a chiral carbon are assigned priority, based primarily on the atomic number of the atom directly bonded to the carbon atom -I -Br -Cl Atom or Group Reason for Priority: First Point of Difference (Atomic numbers) bromine (35) chlorine (17) iodine (53) -OH -NH2 -SH O Cl oxygen (8) nitrogen (7) sulfur(16) chlorine (17) O -COH O -CNH2 O carbon to oxygen, oxygen, then oxygen (6 ->8, 8, 8) carbon to oxygen, oxygen, then nitrogen (6 ->8, 8, 7) -CH -CH2 OH -CH2 CH3 -CH2 NH2 carbon to oxygen, oxygen, then hydrogen (6 ->8, 8, 1) carbon to oxygen (6 -> 8) carbon to carbon (6 -> 6) carbon to nitrogen (6 -> 7) 2 3 -CH2 H -H ( ) carbon to hydrogen (6 -> 1) hydrogen (1)
- 5. Absolute Configuration: R and S Notations Each stereogenic center is assigned a configuration based on the following rules Each stereogenic center is assigned a configuration, based on the following rules 1. Use the Cahn-Ingold-Prelog priority rules to assign priority (one through four) to the four groups on the chiral carbon atom. 2. Orient the molecule so that the lowest priority atom is in the back (away from you). Look at the remaining three groups of priority 1-3 If the remaining three groups are Look at the remaining three groups of priority 1-3. If the remaining three groups are arranged so that the priorities 1→2→3 are in a clockwise fashion, then assign the chiral center as R (“rectus” or right). If the remaining three groups are arranged 1→2→3 in a counterclockwise manner, then assign the chiral center as S (“sinister” or left)
- 6. Orienting a Tetrahedron – The Double Switch Interchanging any two groups inverts the stereochemistry. So switch the lowest priority group to the desired position. Then switch any other two groups. The “double-switch” does not change the stereochemistry.
- 7. Fischer Projections Representation of a three-dimensional molecule as a flat structure. A tetrahedral carbon is represented using just two crossed lines: Horizontal line is coming out of the plane of the page (towards you) and vertical line is going back behind the plane of the paper (away from you) F I Cl F Br I Br Br Cl H H HOOC CH3 OH COOH H3C OH
- 8. Manipulation of Fischer Projections Rotating a Fischer projection by 180° retains the configuration Rotating a Fischer projection by 180 retains the configuration Rotating a Fischer projection by 90° inverts the configuration g p j y g If one group of a Fischer projection is held steady, the other three groups can be rotated g p p j y, g p clockwise or counterclockwise without altering the configuration.
- 9. Assigning R and S Configurations to Fischer Projections 1. Assign priorities to the four substitutents according to the Cahn-Ingold-Prelog rules 2. Perform the two allowed manipulations of the Fischer projection to place the lowest priority group at the top (or bottom). 3 If the priority of the groups 1→2→3 are clockwise then assign the center as R if 3. If the priority of the groups 1→2→3 are clockwise then assign the center as R, if 1→2→3 are counterclockwise then assign the center as S. CH2CH3 H OH1 2 4 place at the top H HO CH CH 1 2 4 CH3 H OH1 3 4 hold steady CH3 HO CH2CH3 1 2 3 clockwise - R hold steady clockwise R
- 10. Molecules with more than one Stereocenter If a molecule has one stereocenter it exists as R and S isomers, which are enantiomers. If a molecule has two stereocenters, each of them can exist as R and S, independent of the other center. The maximum number of stereoisomers for a molecule having n stereocenters is 2n 2,3-dibromopentane has 2 chiral centers. There can be 4 stereoisomers, which are (2R,3R) (2R 3S) (2R,3S) (2S,3R) (2S,3S) (2R,3R) and (2S,3S) isomers are enantiomers, so are (2R,3S) and (2S,3R) isomers (2R,3R) isomer is a diastereomer of (2R,3S) and (2S,3R) isomers Similarly, (2S,3S) isomer is a diastereomer of (2R,3S) and (2S,3R) isomers
- 11. Stereoisomers of 2,3-dibromopentane H Br CH3 R Br H CH3 S H Br Br H CH2CH3 S Br H H Br CH2CH3 R ers 2 3 2 3 CH CH iastereome Br H CH3 Br H S S H Br CH3 H Br R R Di CH2CH3 CH2CH3 E i Enantiomers
- 12. Stereoisomers of 2,3-dibromobutane CH3 CH3 H Br Br H CH S R Br H H Br CH R S Here the RS and SR isomers are identical molecules. This diastereomer is called ‘meso’ and CH3 CH3 This diastereomer is called ‘meso’ and is an achiral molecule This results from the plane of Br H CH3 B H S S H Br CH3 H B R R symmetry present in this isomer. Thus, although the maximum number of stereoisomers can never be more Br H CH3 S H Br CH3 of stereoisomers can never be more than 2n, the actual number could be lower. Enantiomers
- 13. Meso diastereomers HO OH H H HO OH H H HO OH H H HO H HOOC COOH H H HO OH HO H HOOC COOH Cl Cl Cl Cl
- 14. Chirality Without a Stereocenter - Biphenyls If X is a small group the single bond connecting the two phenyl rings would undergo If X-is a small group, the single bond connecting the two phenyl rings would undergo easy rotation and result in racemization Chirality resulting from restricted rotation about a single bond is called Atropisomerism
- 15. Chi li Wi h S All Chirality Without a Stereocenter - Allenes
- 16. Chi li Wi h S S i C d Chirality Without a Stereocenter – Spiro Compounds