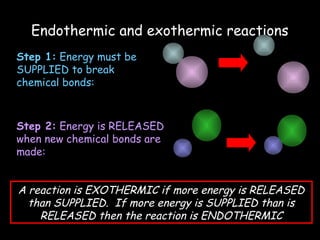
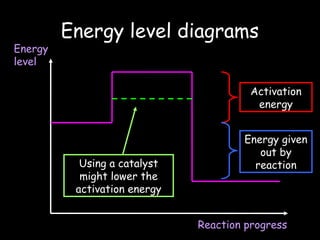
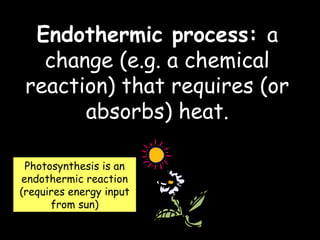
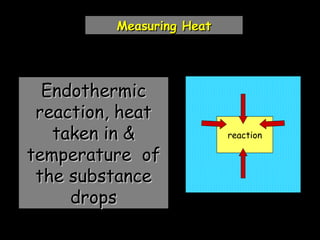
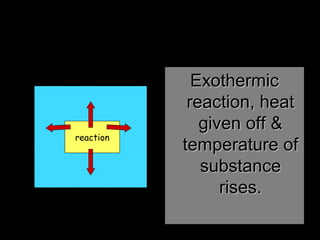
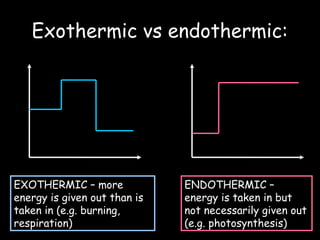
The document discusses endothermic and exothermic reactions. It defines endothermic reactions as those that require energy input to occur, while exothermic reactions release energy. Specific examples are given of endothermic processes like photosynthesis and forming NaCl ions, and exothermic processes like burning fossil fuels. Methods for measuring the temperature change of reactions to determine if they are endothermic or exothermic are also outlined.