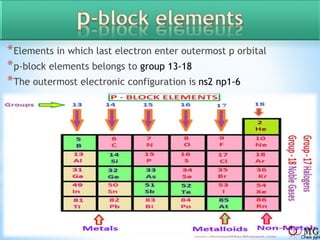
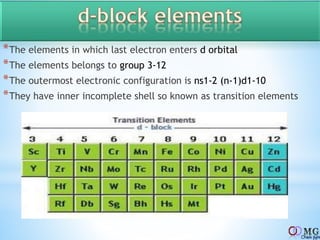
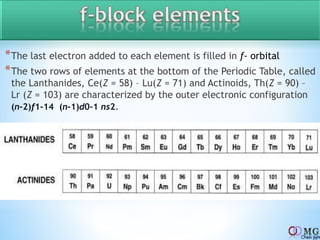
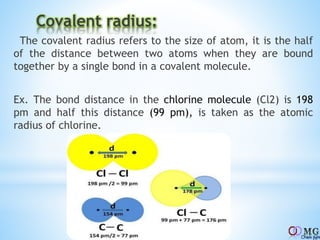
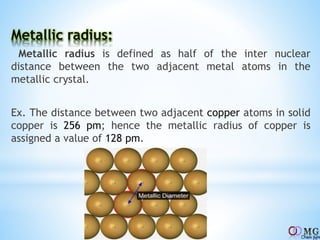
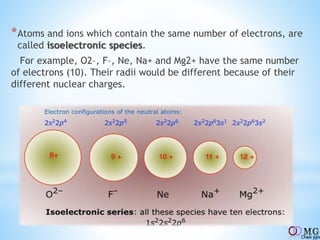
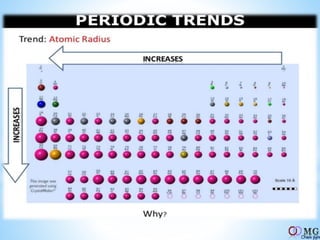
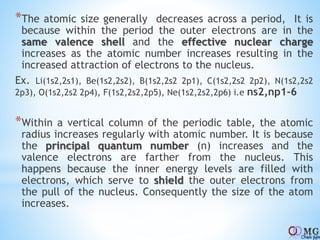
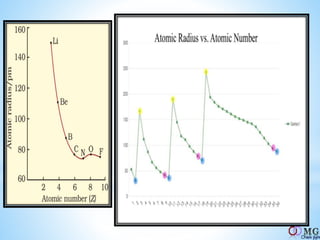
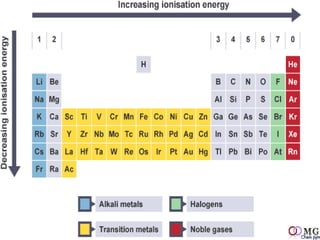
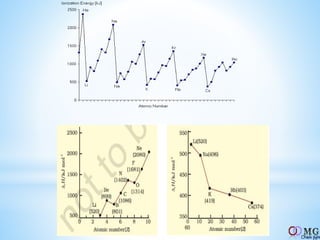
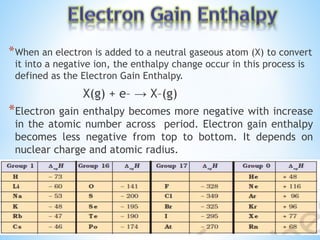
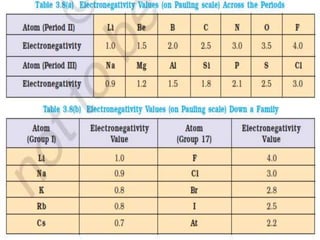
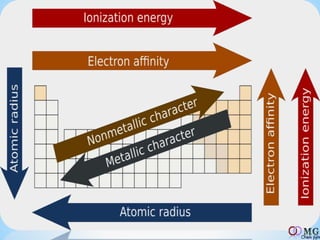
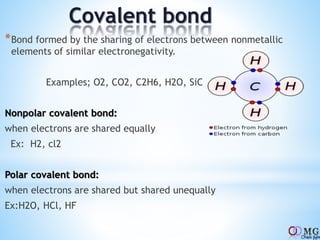
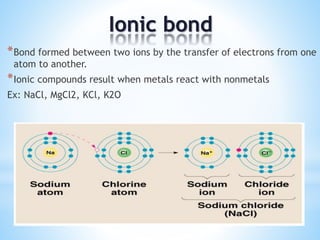
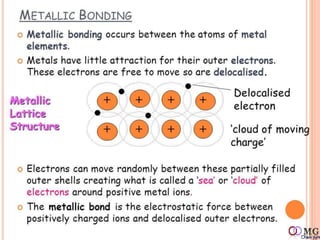
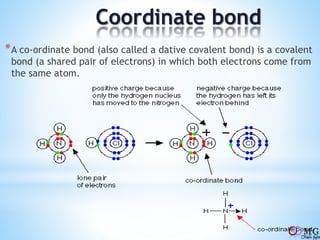
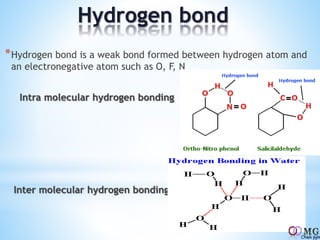
Atomic radii decrease across a period as nuclear charge increases. Cations are smaller than their parent atoms. Among isoelectronic species, the one with the larger positive nuclear charge will have the smallest radius. Ionization energy generally increases across a period as it is more difficult to remove electrons, and decreases down a group as shielding increases. Electronegativity follows similar trends as ionization energy.