Periodicity refers to the recurring trends in properties seen when elements are arranged in order of increasing atomic number in the periodic table. Elements within the same group have similar properties. The periodic table is organized into rows and columns, with elements in the same period having electron shells filling in a similar way.
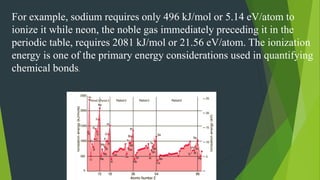
Some key periodic properties that were discussed include atomic radius, ionization energy, electron affinity, and electronegativity. Atomic radius decreases left to right within a period as protons increase, but increases down a group as electron shielding effects occur. Ionization energy generally increases left to right as it takes more energy to remove electrons from smaller atoms. Electron affinity values increase left to right and decrease down a group. Electrone