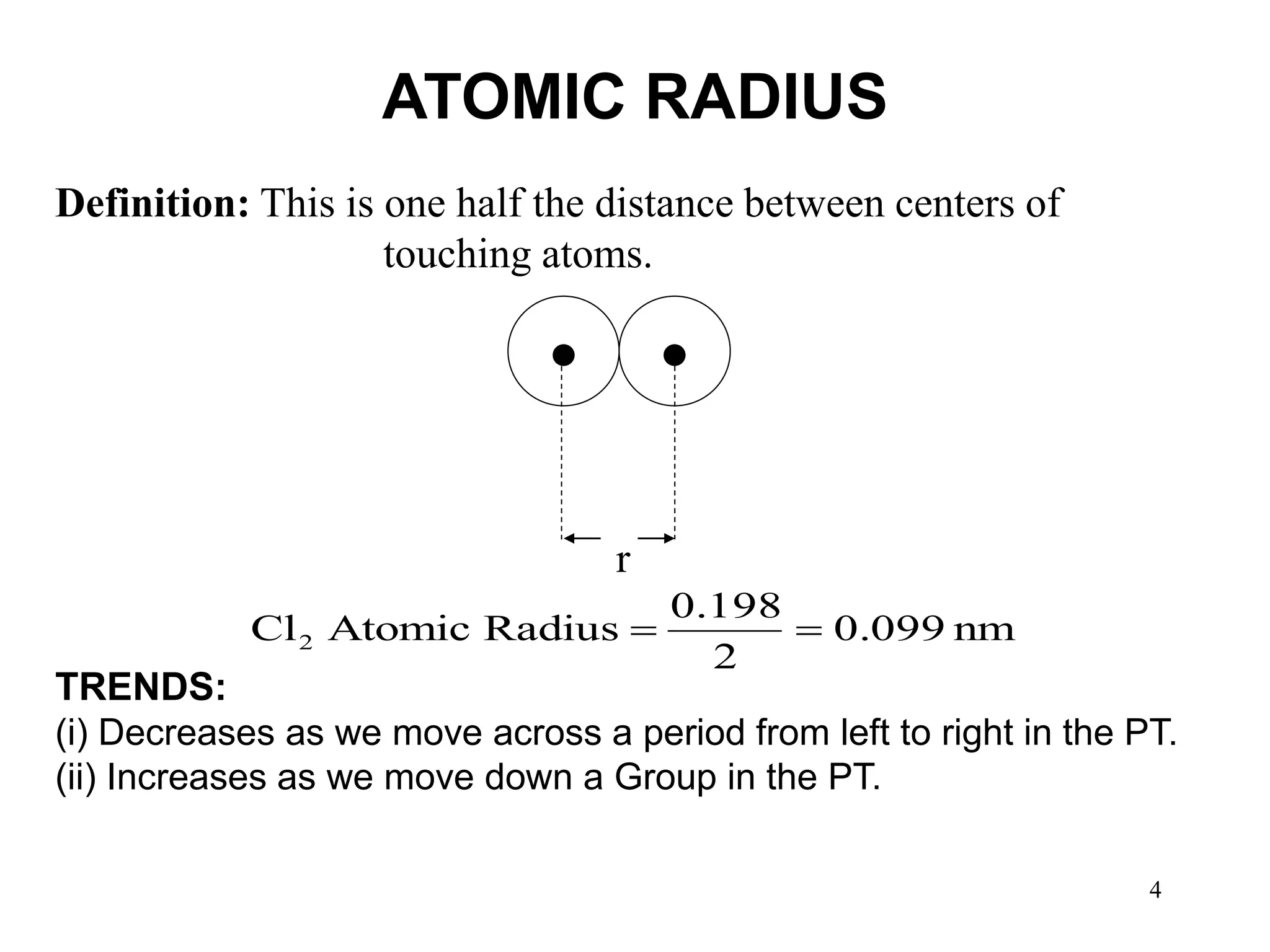
The document explains the structure of the periodic table, categorizing elements into representative, transition, and inner transition elements. It outlines periodic properties such as atomic radius, ionization energy, electron affinity, and electronegativity, including their trends across periods and down groups. Significant definitions and trends are also provided, such as that atomic radius decreases across a period and increases down a group, while ionization energy and electronegativity generally increase across a period and decrease down a group.






![9
ELECTRON AFFINITY
Definition: The energy change that occurs when an electron is
added to a gaseous atom or ion.
i.e. (i) M(g) + e- M-
(g) [Neutral atom]
(ii) M+
(g) + e- M(g) [an ion of charge +1]
TRENDS:
(i) Across a Period
In general electron affinities become more negative (stronger
attraction for an electron) from LEFT to RIGHT of the PT.](https://image.slidesharecdn.com/1periodicproperties-230430081405-06e0c3cf/75/1Periodic-Properties-ppt-9-2048.jpg)


