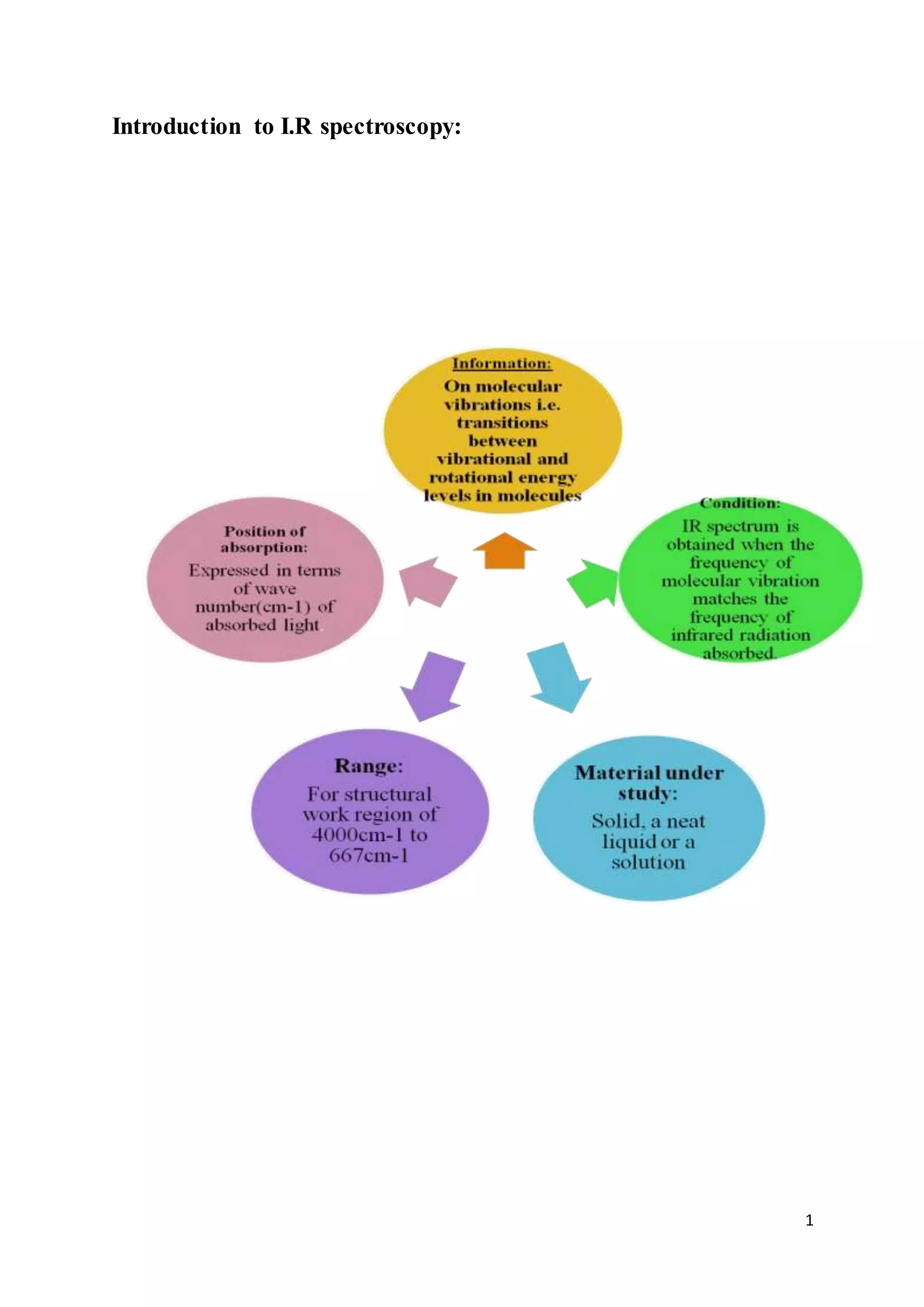
1. Infrared spectroscopy involves measuring the absorption of infrared radiation by a sample and plotting it as a function of wavelength or wavenumber.
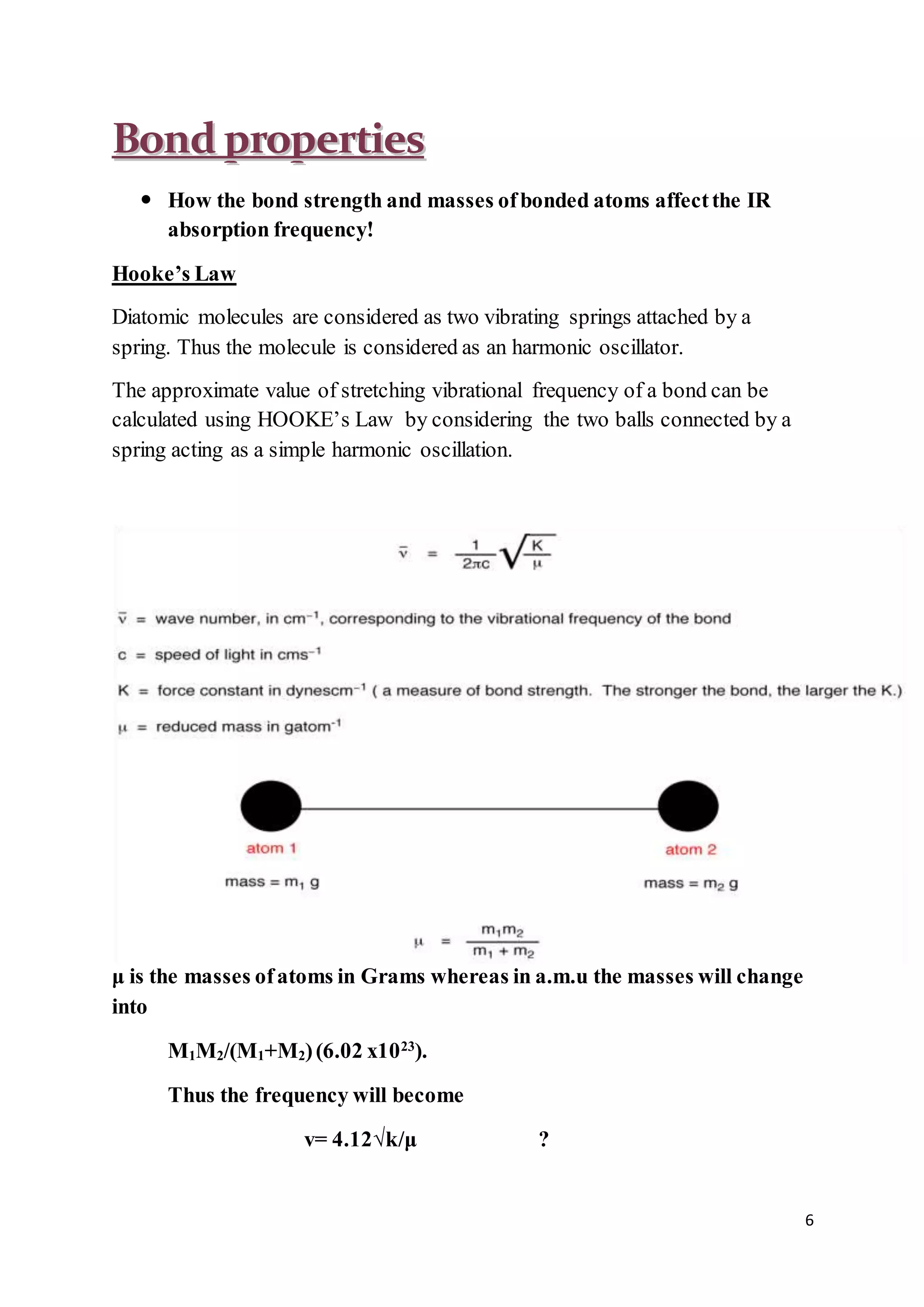
2. Infrared radiation causes transitions between vibrational and rotational energy levels in molecules, allowing the characteristic vibrations of bonds to be observed.
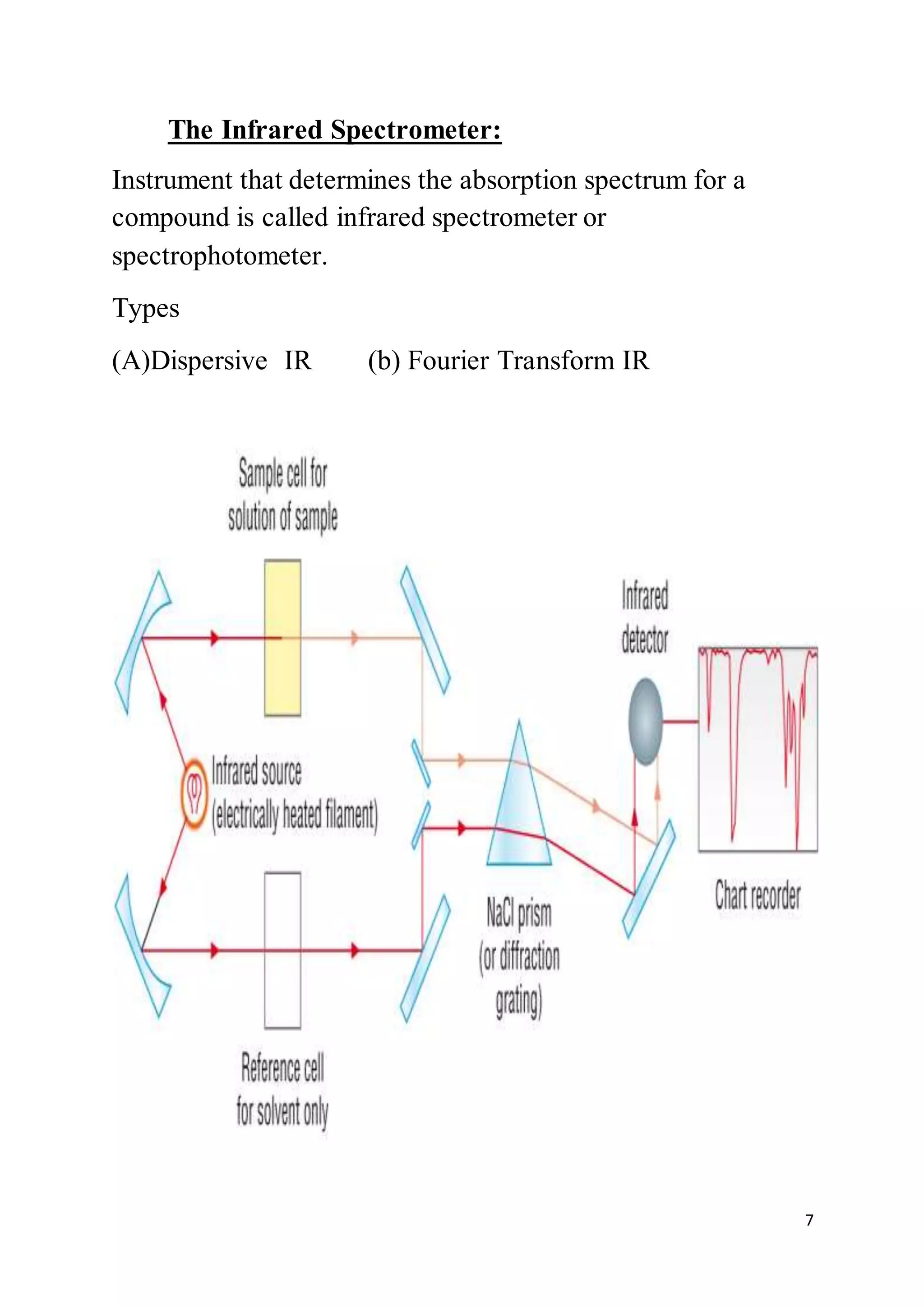
3. An infrared spectrometer consists of an infrared source, sample holder, monochromator to separate wavelengths, detector, and recorder. It measures the infrared absorption spectrum of a sample.