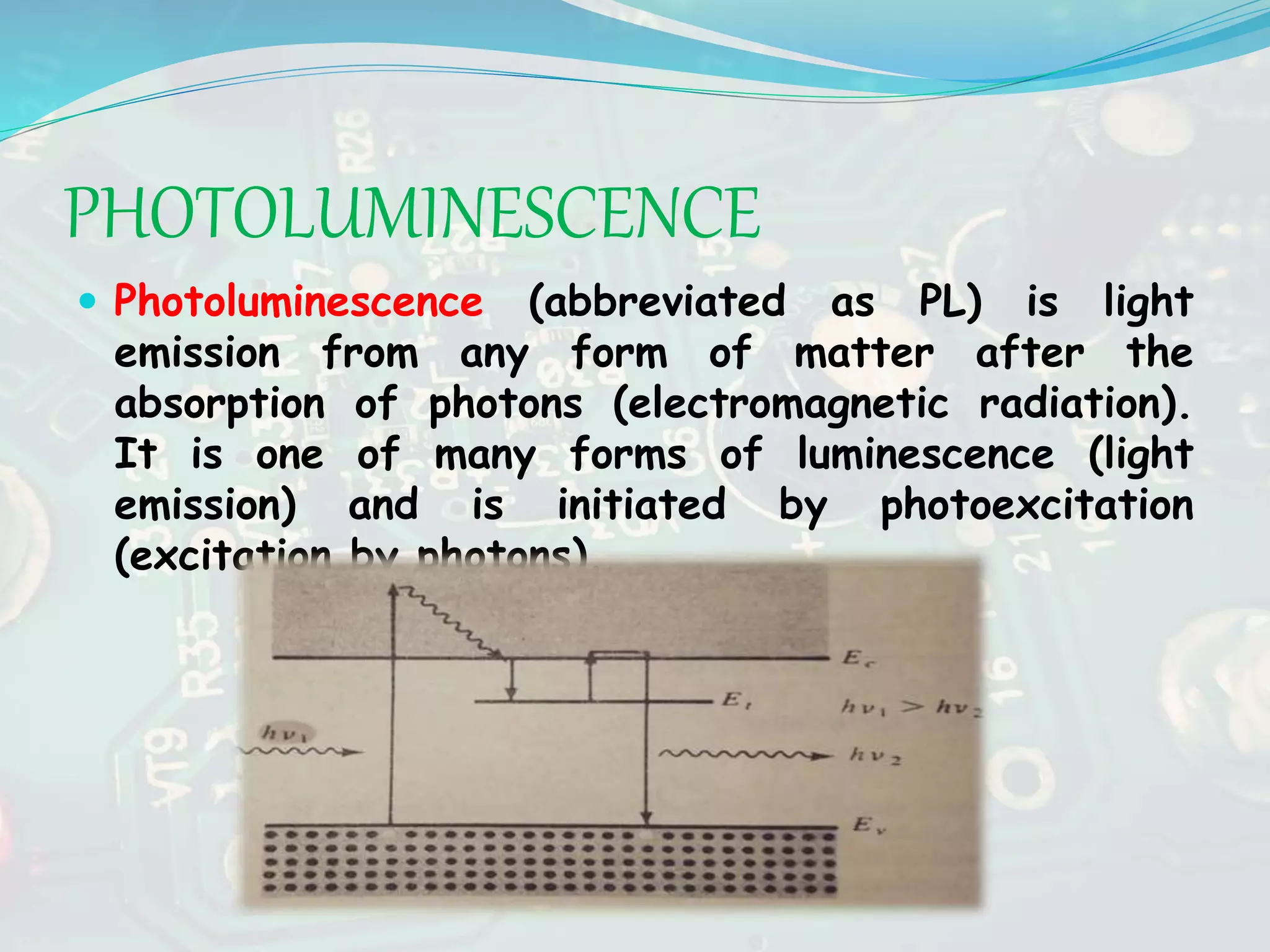
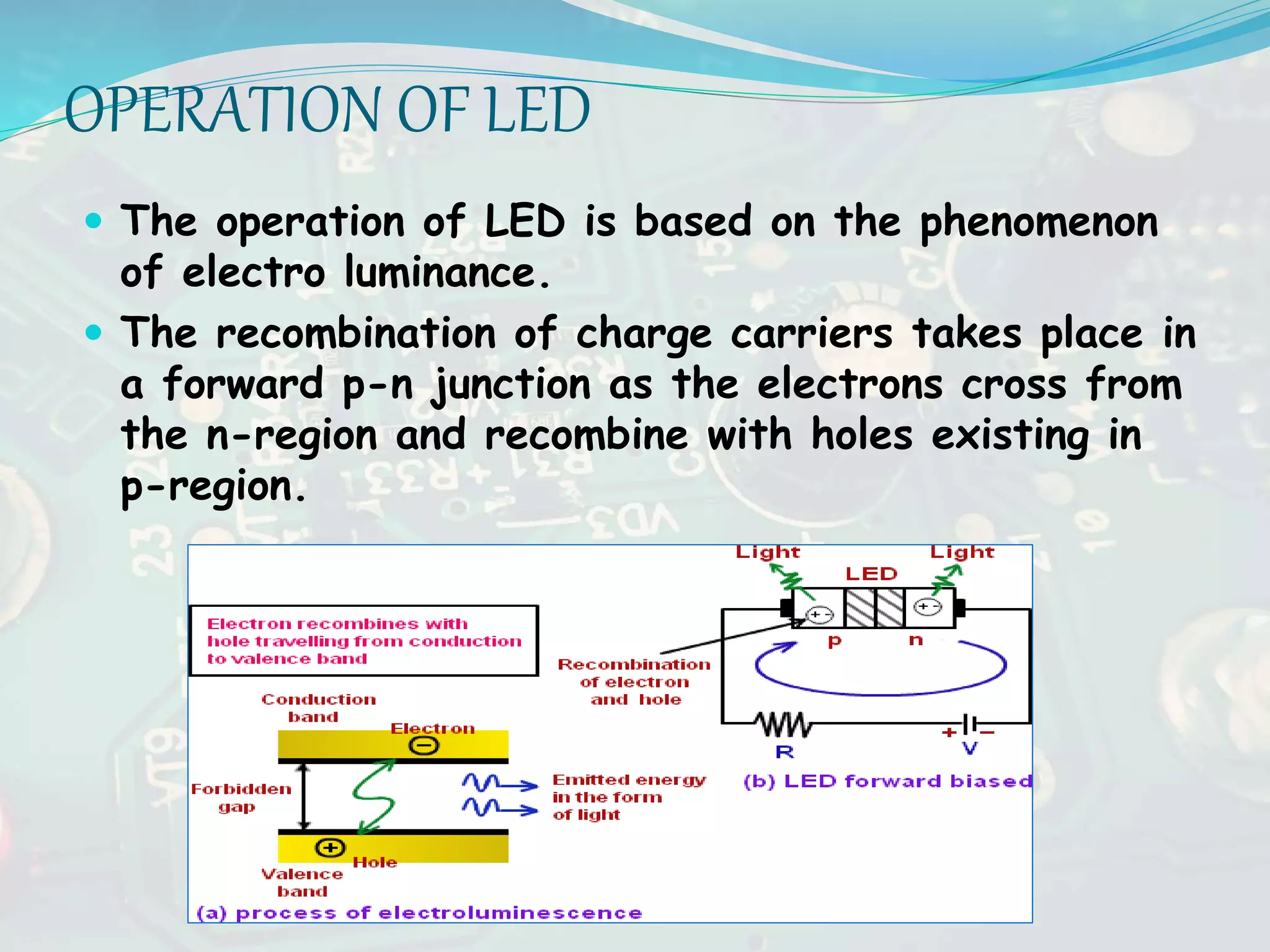
The seminar discussed luminescence and various light-emitting devices. It defined luminescence as light emission not resulting from heat and described different types including photoluminescence, electroluminescence, and cathodoluminescence. It also explained the working principles of light-emitting diodes (LEDs) and how direct bandgap semiconductors allow for light emission. Additional topics covered included solar cells and their use in applications such as toys, watches and water pumping.