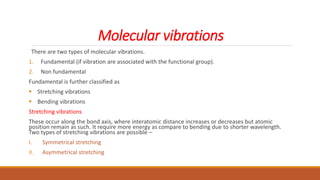
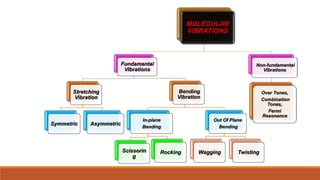
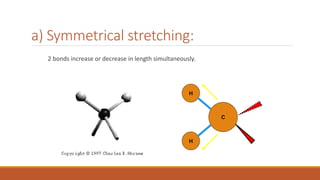
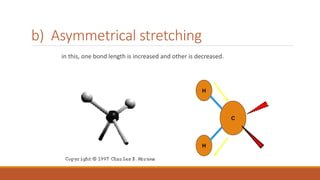
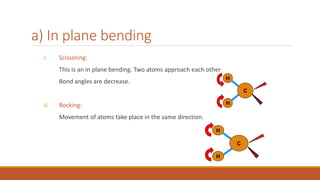
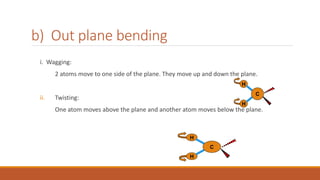
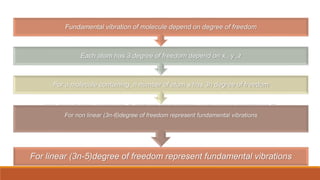
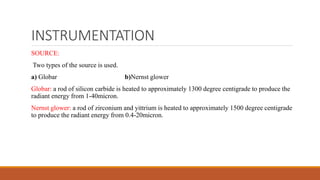
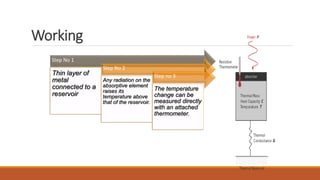
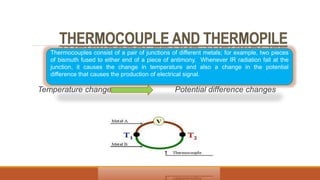
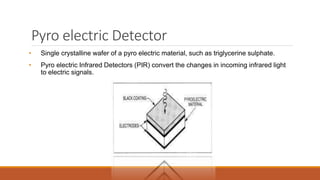
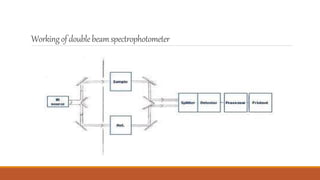
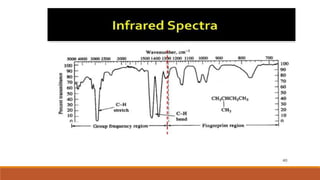
This document provides an overview of infrared spectroscopy. It begins with an introduction to the infrared region of the electromagnetic spectrum and the principle of IR spectroscopy, which is that IR radiation causes excitation of molecules between vibrational energy states. It then discusses molecular vibrations including stretching and bending vibrations. The document also covers instrumentation components such as sources, detectors, and the working of IR spectrometers. Finally, it lists several applications of IR spectroscopy including identification of substances, determination of molecular structure, detection of impurities, and following reaction progress.