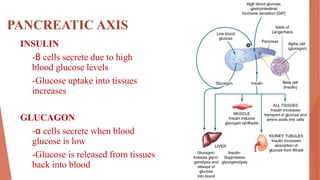
This document discusses insulin and antidiabetic drugs. It begins by describing the pancreatic axis and role of insulin and glucagon in maintaining glucose homeostasis. It then defines diabetes mellitus and describes the main types, Type 1 and Type 2 diabetes. It discusses treatment approaches for both types, including lifestyle changes and various drug classes. The mechanisms and preparations of insulin are outlined in detail. Finally, it reviews common oral antidiabetic drugs like sulfonylureas, meglitinides, biguanides, thiazolidinediones, and alpha-glucosidase inhibitors.