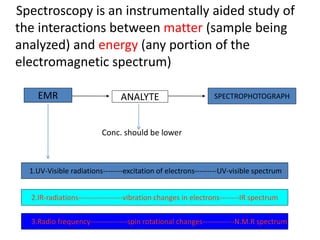
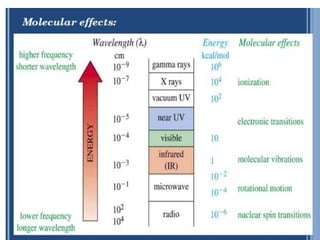
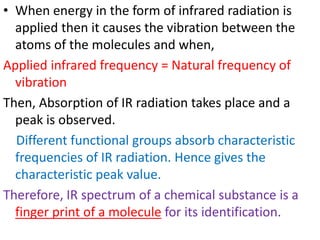
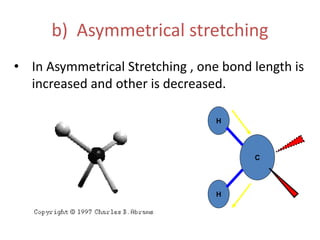
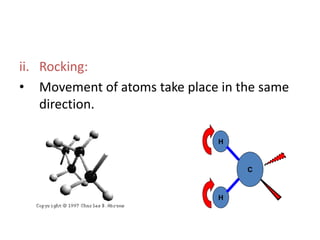
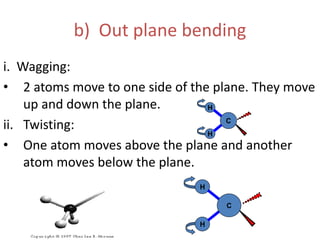
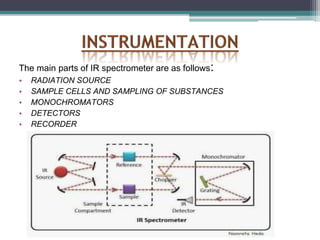
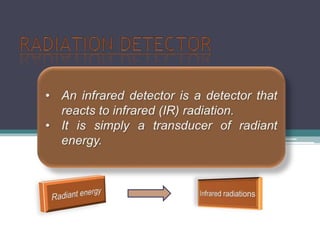
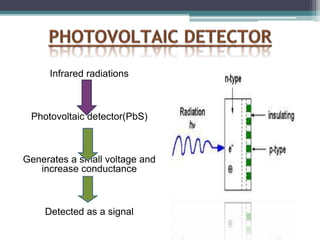
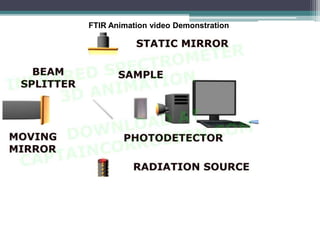
IR spectroscopy analyzes molecular vibrations and rotations that are excited by infrared light. When the frequency of infrared light matches the natural vibrational frequency of a molecule, absorption occurs. Different functional groups absorb characteristic frequencies allowing IR spectroscopy to determine a molecule's structure. A proper sampling technique like mulling or using a liquid cell is required to obtain an IR spectrum. An IR spectrometer consists of an infrared source, monochromator, sample holder, detector, and recorder. Common detectors include bolometers and pyroelectric detectors which convert the infrared absorption into electrical signals.