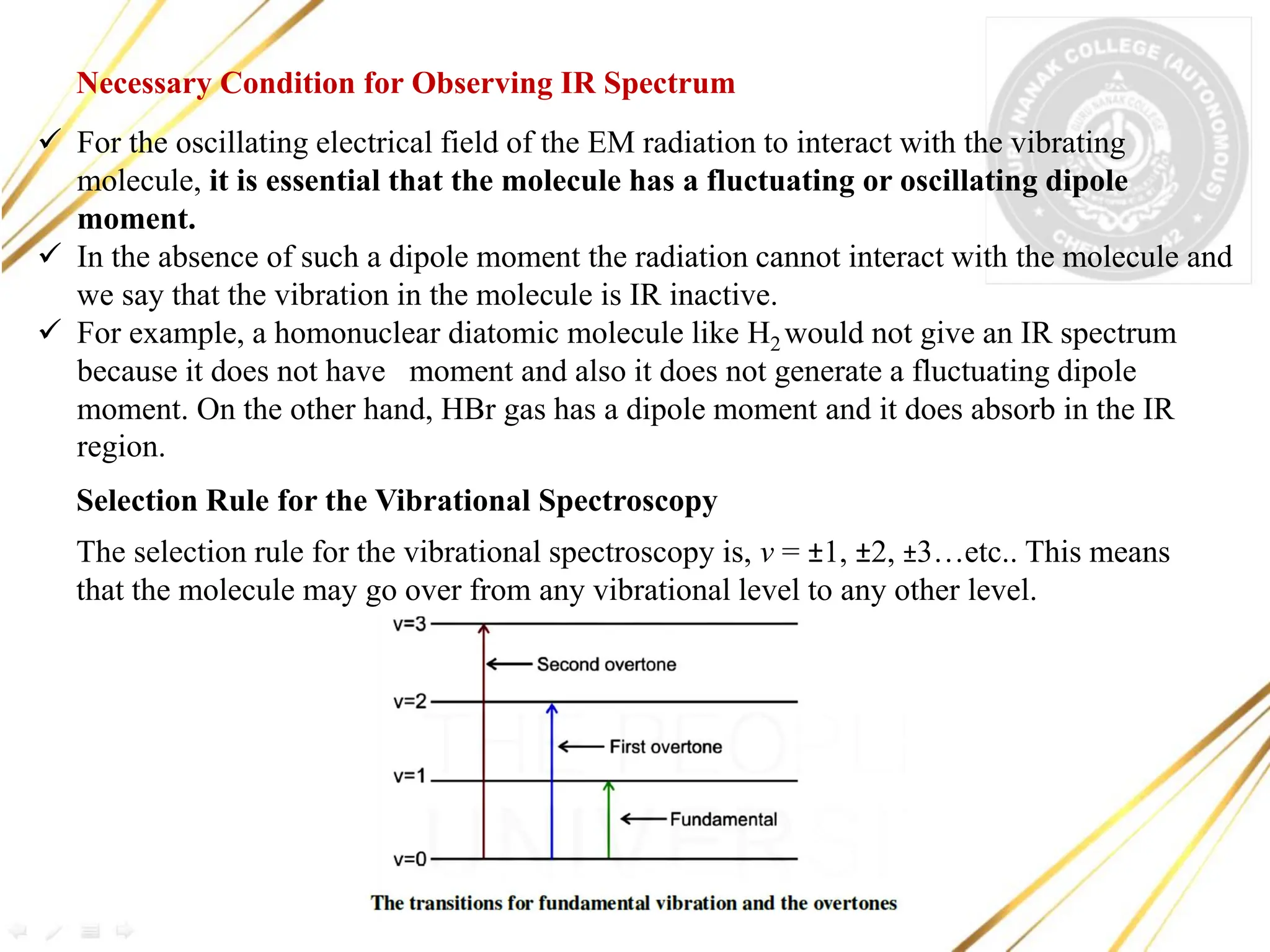
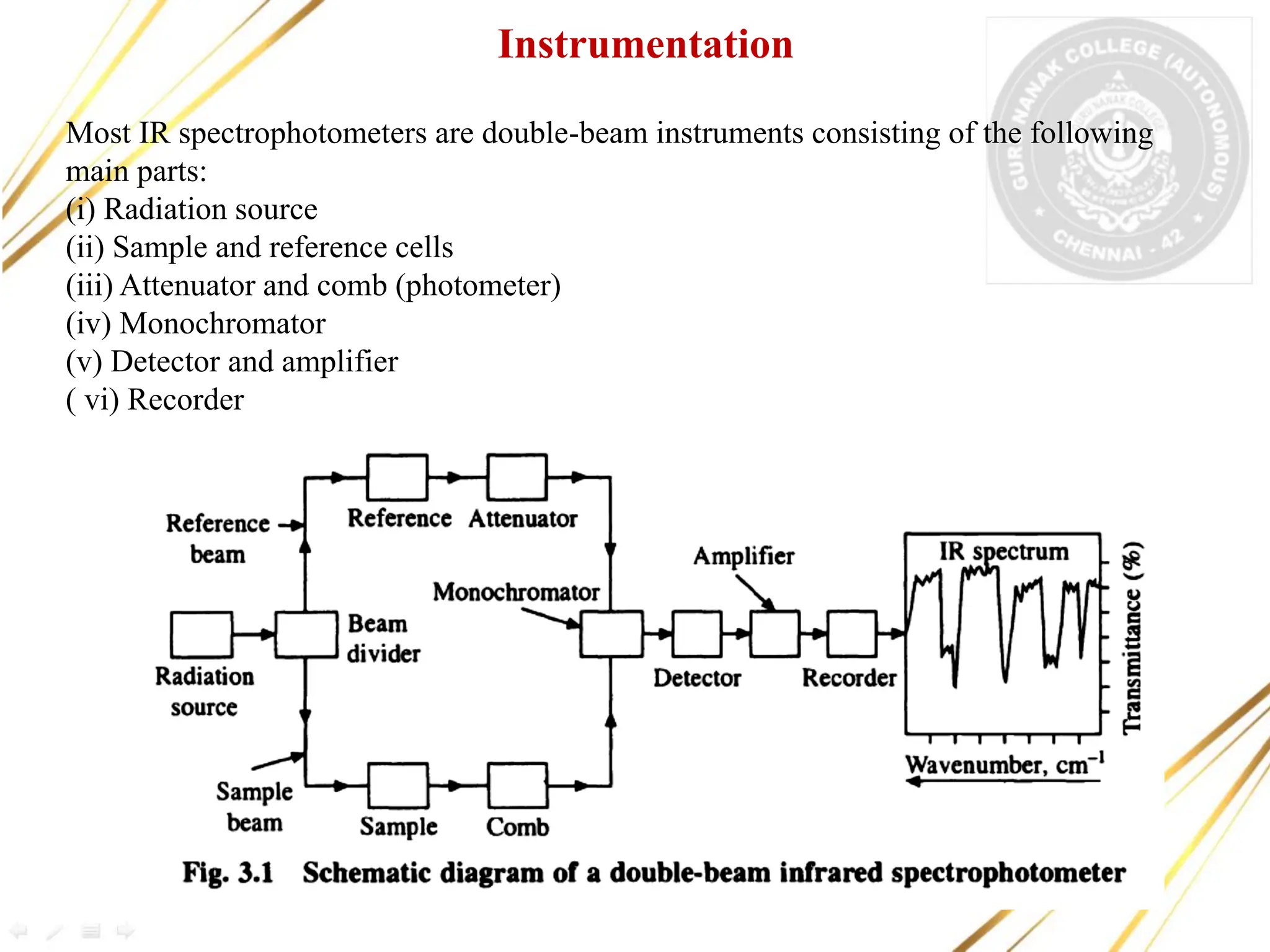
Infrared spectroscopy deals with the absorption of infrared radiation by molecules and the recording of absorption spectra. IR spectra provide information about the types of bonds in a molecule from the region of absorption. The document discusses the principles of IR spectroscopy, instrumentation, types of molecular vibrations observed in IR spectra, and applications such as detection of functional groups, identification of compounds, and study of hydrogen bonding and reaction progress.