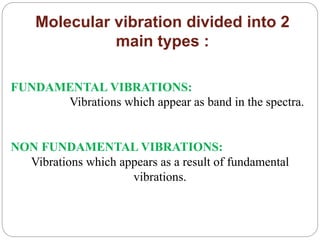
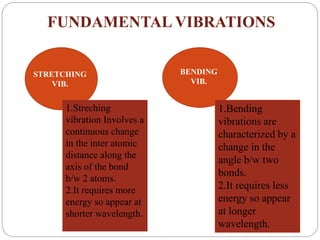
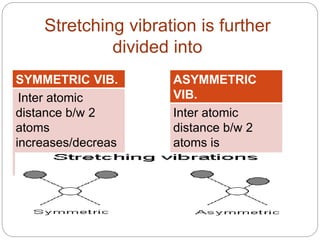
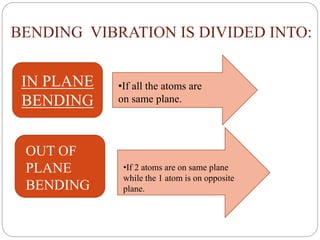
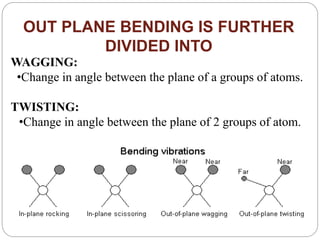
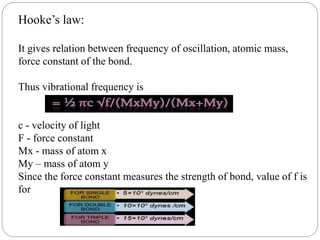
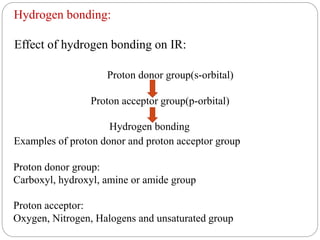
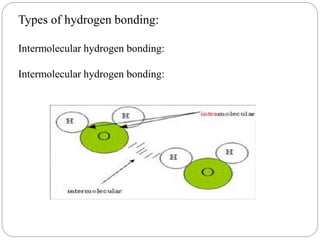
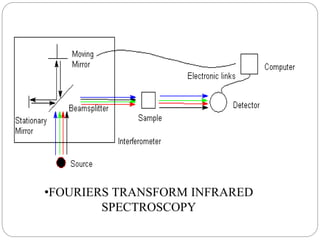
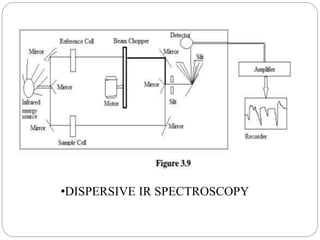
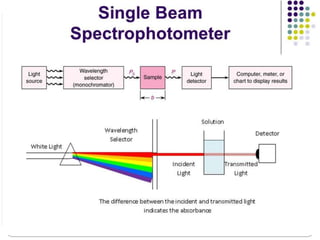
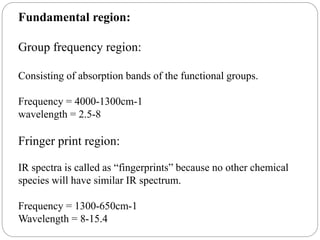
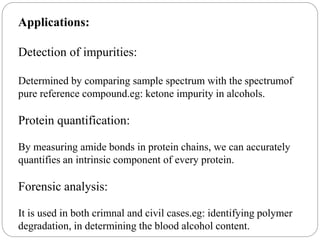
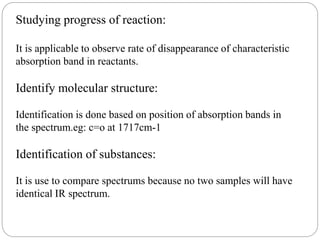
This document provides an overview of infrared spectroscopy (IR) and its applications. It discusses the IR regions, the principles of IR which involve molecular vibrations and interactions with IR radiation. It describes fundamental vibrations such as stretching and bending, and instrumentation used including sources, sample handling, and detectors. Fourier transform IR is highlighted as a preferred method. Applications include detection of impurities, protein quantification, forensic analysis, and studying reaction progress and molecular structure identification.