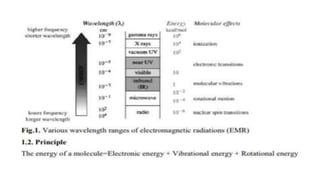
The document discusses infrared spectroscopy, including its principles, types of vibrations (coupled vibrations, Fermi resonance, electronic effects, and hydrogen bonding), and applications in organic chemistry like substance identification and molecular structure determination. It details instrumentation used, such as various detectors and the advantages of FT-IR, including improved signal quality and application versatility. Limitations of IR spectroscopy and the significance of sample preparation are also covered.