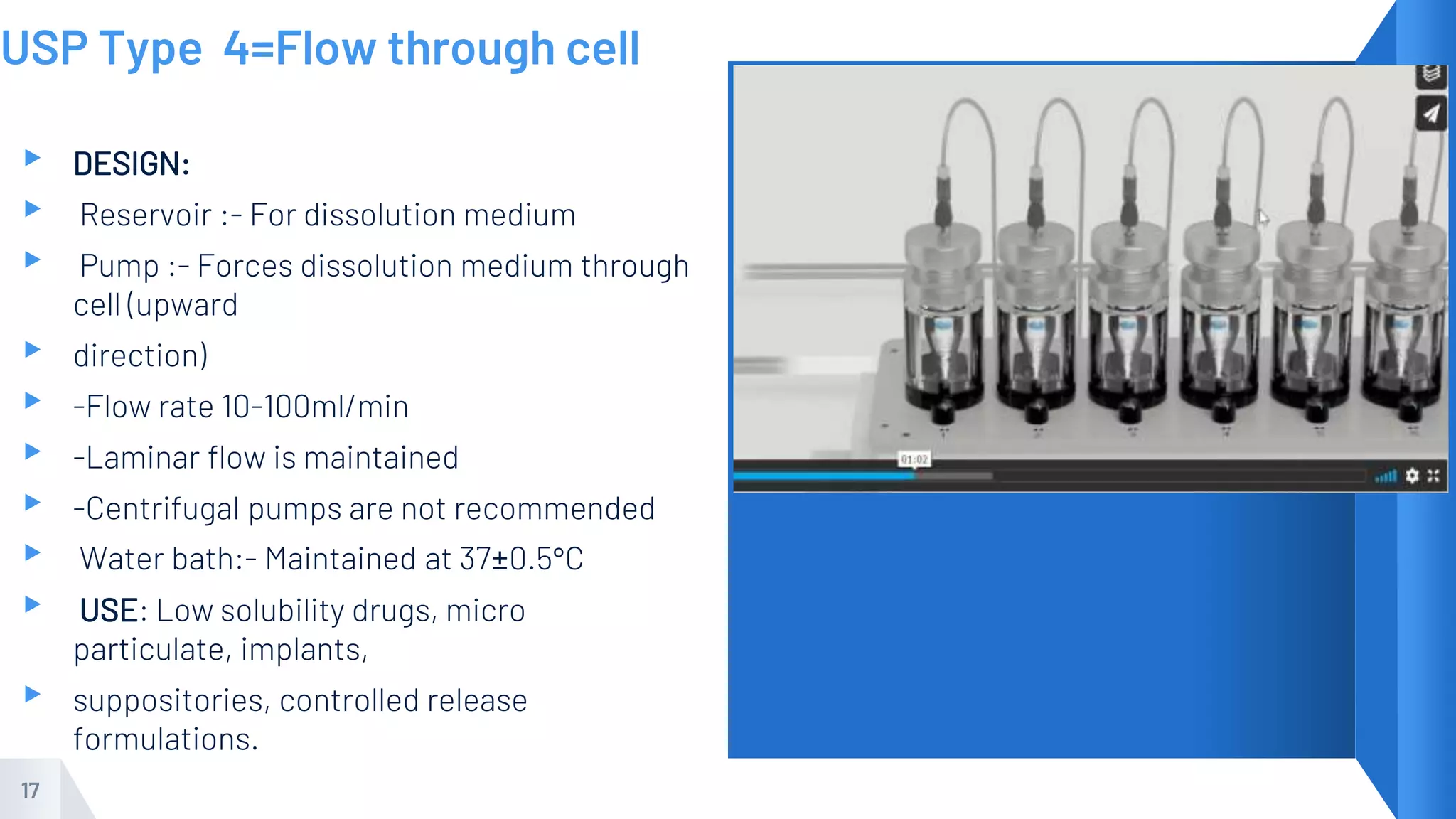
This document provides an overview of dissolution testing. It defines dissolution as the process by which a solid solute enters into a solution. The document discusses the importance of dissolution studies for quality control and product development. It describes the common types of dissolution apparatus, including baskets, paddles, reciprocating cylinders, and flow-through cells. The document also discusses biopharmaceutics classification systems and factors to consider for developing dissolution methods such as choice of medium, solubility, and stability testing.