This document provides information on imaging of aortic dissection, including:
1. CT is the most sensitive imaging method for detecting aortic dissection, showing features like an intimal flap separating the true and false lumens.
2. Risk factors include hypertension, male sex, advanced age, and connective tissue disorders. Acute aortic dissections are classified as Stanford type A or B.
3. Management decisions are based on details of the dissection like entry/exit points and branch vessel involvement that affect outcomes.



![Aortic Dissection (AD) definition
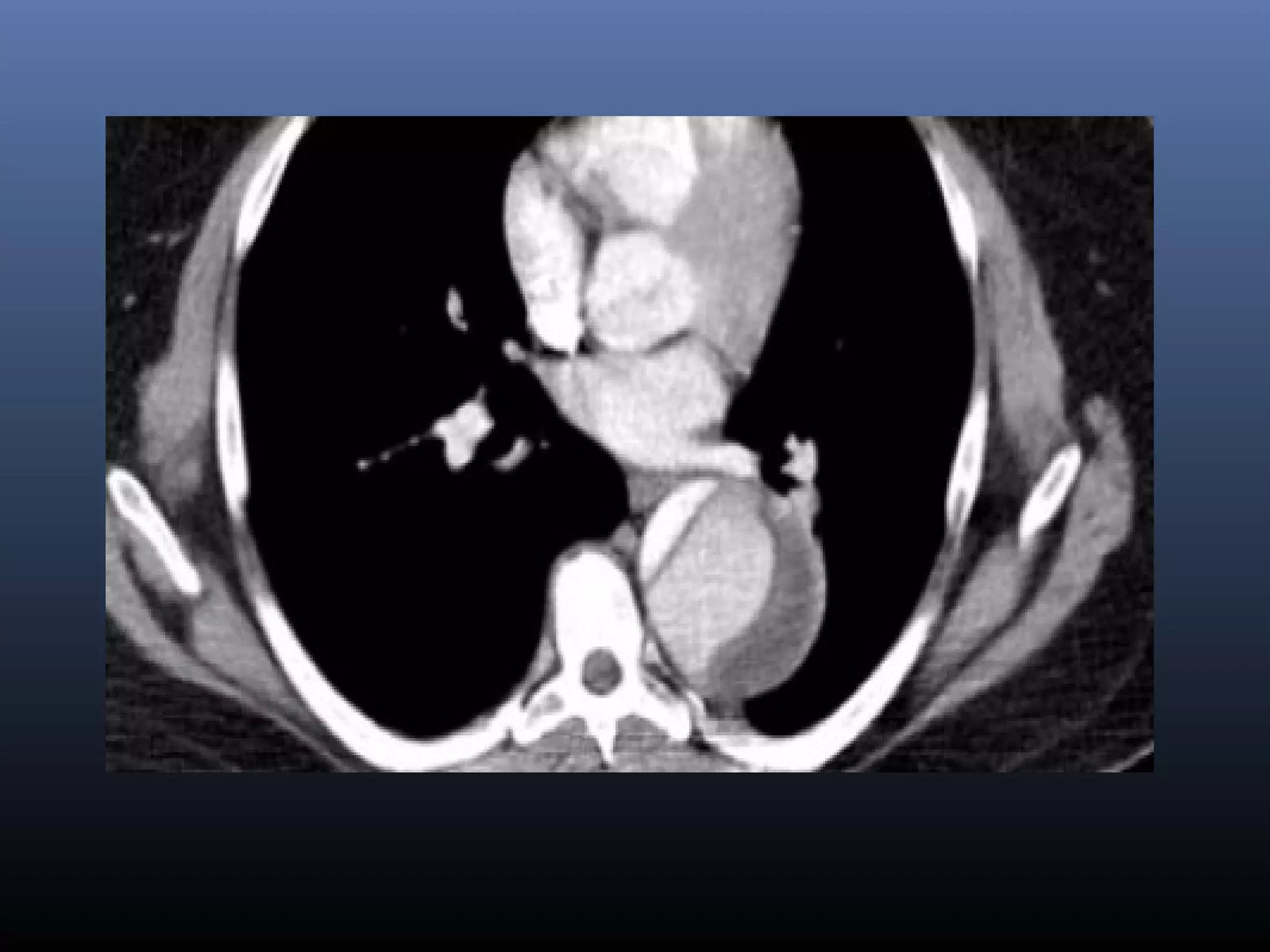
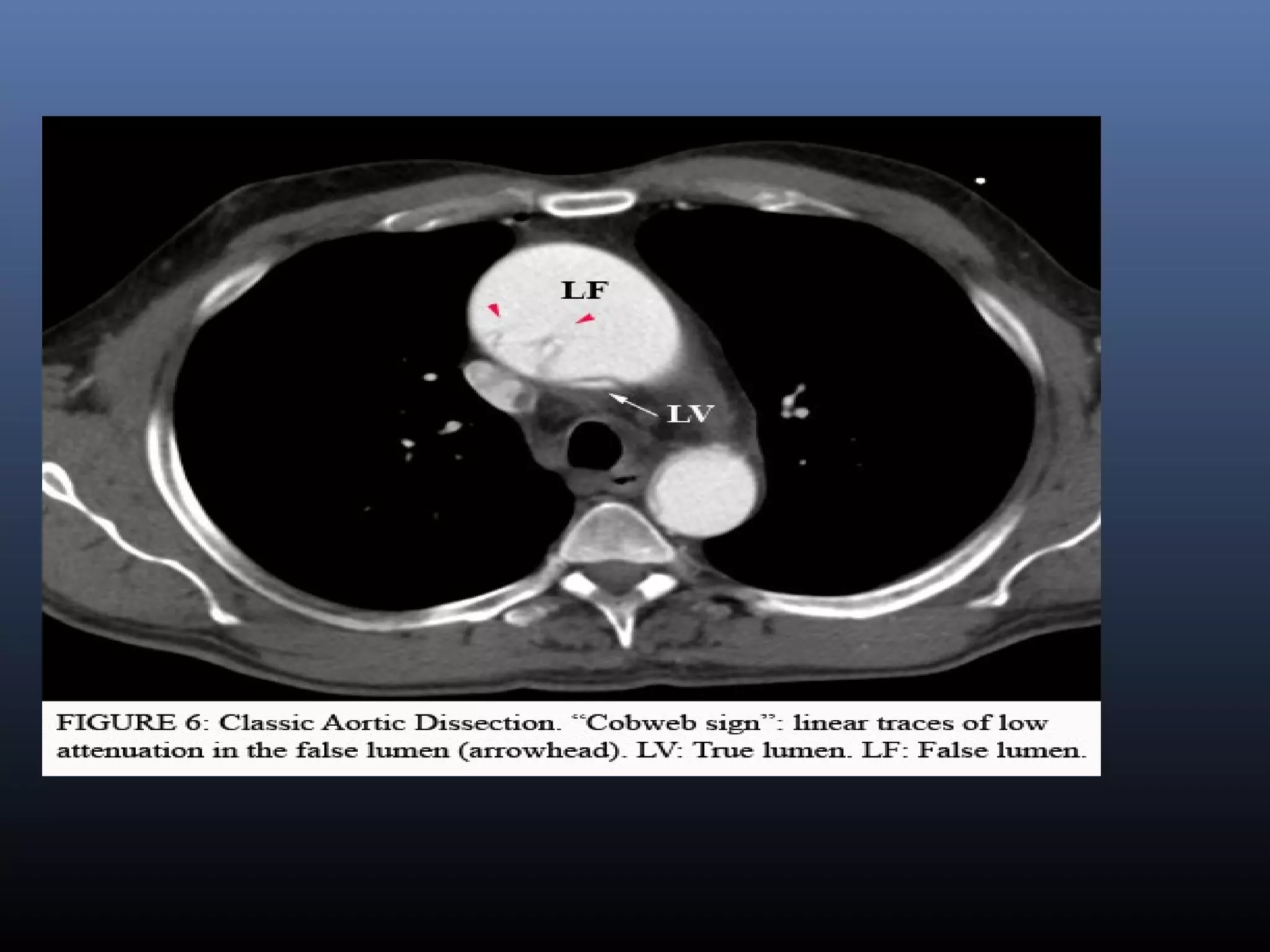
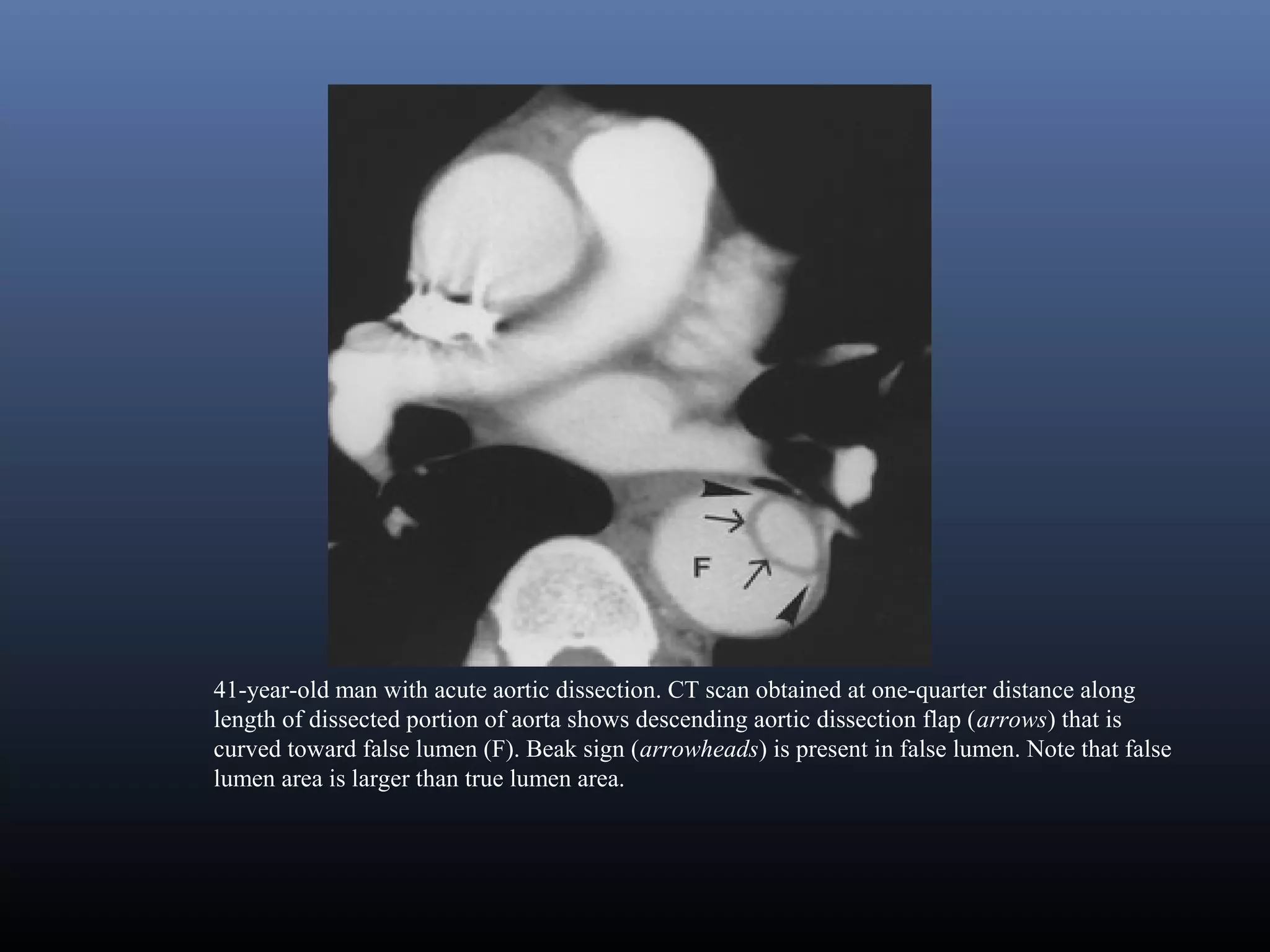
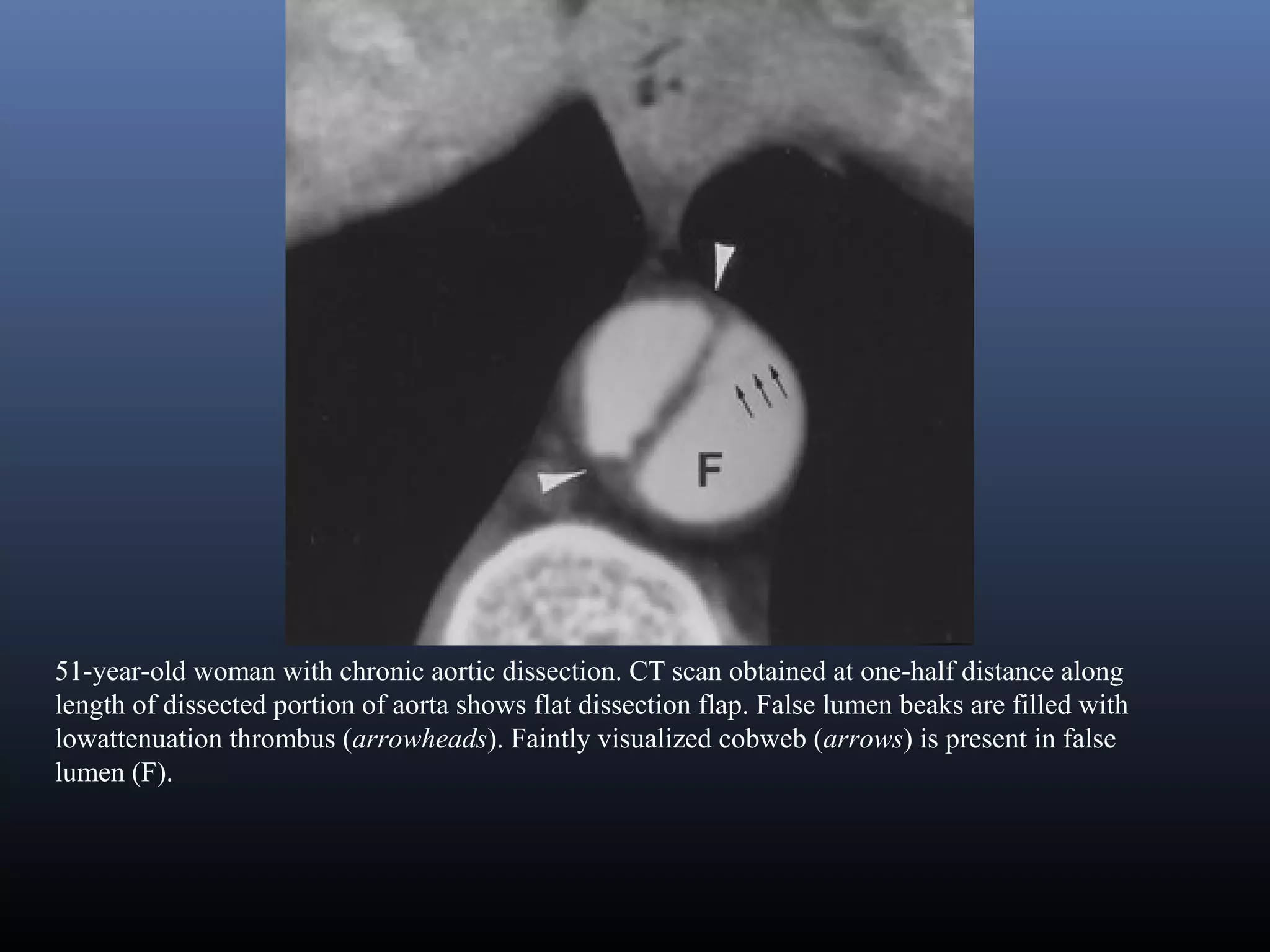
Classic aortic dissection is a longitudinal split or partition in the media of the aorta. An intimal tear
connects the media with the aortic lumen, and an exit tear creates a true and a false lumen. The smaller
true lumen is lined by intima, and the false lumen is lined by media. Typically, flow in the false lumen
is slower than in the true lumen, and the false lumen often becomes aneurysmal when subjected to
systemic pressure. An acute aortic dissection is considered chronic at 2 weeks. The dissection usually
stops at an aortic branch vessel or at the level of an atherosclerotic plaque.[1, 2]
Diagram illustrates events leading to aortic dissection from formation of entrance tear and exit tear of intima to splitting of aortic media and
formation of intimomedial flap. Blood under pressure dissects media longitudinally, and double-channel aorta is formed with blood filling both
true and false lumens.](https://image.slidesharecdn.com/aorticdissection-160113083845/75/Imaging-of-Aortic-Dissection-5-2048.jpg)


![Signs and symptoms
Sudden onset of severe chest pain that often has a tearing or ripping quality (classic symptom)
Chest pain may be mild
Anterior chest pain: Usually associated with anterior arch or aortic root dissection
Neck or jaw pain: With aortic arch involvement and extension into the great vessels
Tearing or ripping intrascapular pain: May indicate dissection involving the descending aorta
No pain in about 10% of patients
Syncope
Cerebrovascular accident (CVA) symptoms (eg, hemianesthesia, and hemiparesis, hemiplegia) [1]
Altered mental status
Numbness and tingling, pain, or weakness in the extremities
Horner syndrome (ie, ptosis, miosis, anhidrosis)
Dyspnea
Hemoptysis
Dysphagia
Flank pain (with renal artery involvement
Abdominal pain (with abdominal aorta involvement)
Fever
Anxiety and premonitions of death](https://image.slidesharecdn.com/aorticdissection-160113083845/75/Imaging-of-Aortic-Dissection-8-2048.jpg)