This document discusses two case studies related to stability failures and deficiencies in stability programs.
Case Study 1 describes a product where the gelatin source was changed without properly assessing the impact, resulting in stability failures and the recall of hundreds of batches. The factory was shut down for years and people lost their jobs.
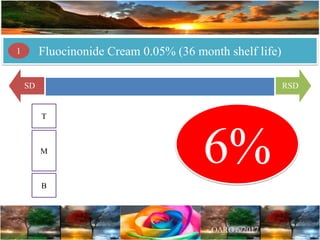
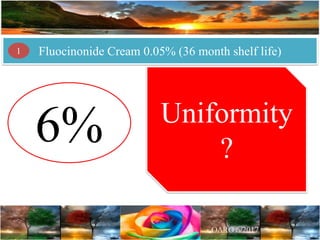
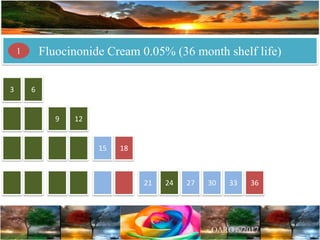
Case Study 2 describes a fluocinonide cream that failed the 6-month stability testing for 3 out of 24 lots. This calls into question the correctness of the product's 36-month shelf life determination and stability program.
The document emphasizes the importance of characterizing natural materials like gelatin given their variability, and having robust stability programs to avoid regulatory issues.