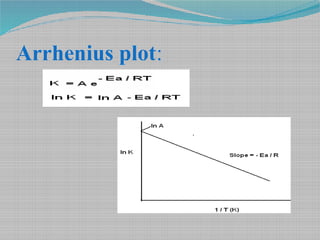
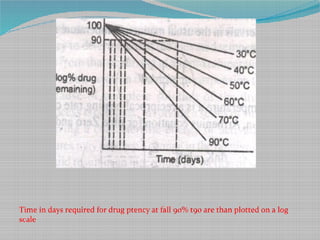
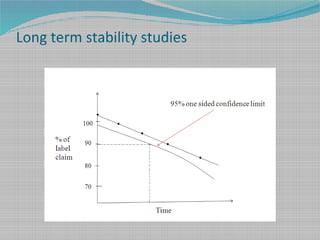
Drug stability refers to the extent to which a pharmaceutical product retains its quality attributes, such as concentration of active ingredients, over time. Stability testing is necessary to determine a drug's shelf life and recommended storage conditions. It involves evaluating a drug's chemical, physical, and microbial properties under different temperatures and humidity levels over time. The Arrhenius equation can be used to predict a drug's stability at normal temperatures based on its degradation rates observed during accelerated stability testing at elevated temperatures. International guidelines provide recommendations for long-term and accelerated stability study protocols and minimum data requirements for drug substances and products to ensure quality, safety and efficacy over a product's shelf life.