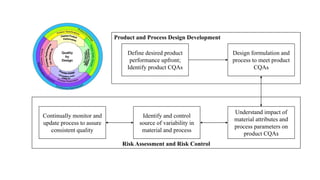
This document provides an overview of ICH Q8 guidelines on pharmaceutical development and quality by design. It discusses key concepts like quality target product profiles, critical quality attributes, risk assessment, design space, control strategy, and continual improvement. The guidelines describe applying a science and risk-based approach to developing pharmaceutical products and manufacturing processes to consistently deliver intended performance. A design space is established based on understanding the impact of material attributes and process parameters on critical quality attributes. This knowledge facilitates more flexible regulatory approaches within the approved design space.