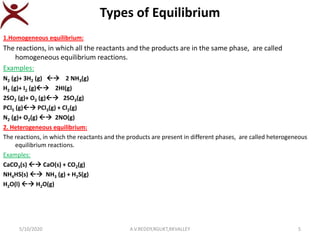
1. The document discusses chemical equilibrium, describing reversible reactions that can proceed in both the forward and backward directions under the same conditions until equilibrium is reached.
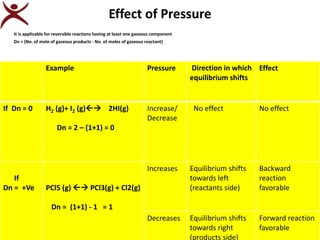
2. Key factors that affect chemical equilibrium are discussed, including concentration, pressure, and temperature based on Le Chatelier's principle. The principle states that if a system at equilibrium experiences a change, the equilibrium shifts to counteract the change.
3. Examples of industrial processes that utilize chemical equilibrium principles are described briefly, including the Haber process for ammonia synthesis and the contact process for sulfur trioxide production.




![Law of mass action
It is proposed by guldberg and waage
According to this rate of chemical reaction is directly proportional to product of
active masses of all reactants which are raised to the power equals to its
stoichiometric coefficients in the balanced equation.
Rate of reaction(r) = change in concentration of R(or)P / change in time
Rate of reaction(r) = DC/Dt
Ex: R P , r = - D[R]/Dt = D[P]/Dt
Active mass(a):
For pure solids, pure liquids a = 1(unity)
(because the active mass of pure solids and liquids depends on the
density and molecular mass. The density and molecular of a mass of
pure liquids and solids are constant)
In case of solutions , a = molar concentration (Molarity)
In case of gases , a = Partial pressure (or) molar concentration
(Molarity)
5/10/2020 A.V.REDDY,RGUKT,RKVALLEY 6](https://image.slidesharecdn.com/chemicalequilibrium-231106060130-2a0d0b67/85/Chemical-Equilibrium-pptx-6-320.jpg)
![Equilibrium Constant
Let us consider a hypothetical reaction
aA + bB cC + dD
according to the law of mass action,
Rate of the forward reaction, rf α [A]a [B]b
or Rate of the forward reaction, rf = kf [A]a [B]b
kf = rate constant of forward reaction
Similarly,
Rate of the backward reaction, rb α [C]c [D]d
or Rate of the backward reaction, rb = kb[C]c [D]d
kb = rate constant of backward reaction
At equilibrium, the two rates become equal, i.e.,
Rate of the forward reaction(rf) = Rate of the backward reaction(rb)
5/10/2020 A.V.REDDY,RGUKT,RKVALLEY 7](https://image.slidesharecdn.com/chemicalequilibrium-231106060130-2a0d0b67/85/Chemical-Equilibrium-pptx-7-320.jpg)
![Equilibrium Constant
So that, at equilibrium
kf [A]a [B]b = kb [C]c [D]d
But, kf / kb = Equilibrium constant, Kc
So,
The above equation is the law of chemical equilibrium.
5/10/2020 A.V.REDDY,RGUKT,RKVALLEY 8](https://image.slidesharecdn.com/chemicalequilibrium-231106060130-2a0d0b67/85/Chemical-Equilibrium-pptx-8-320.jpg)
![Relationshipbetween Kc and Kp:
aA(g) + bB(g) cC(g) + dD(g)
Let us assume A,B,C,D are ideal gases.
From ideal gas equation,
PV = nRT , P = (n/V)RT molar concentration = n/V.
PA = [A]RT , PB = [B]RT ,PC = [C]RT, PD = [D]RT
So that,
∆n = [(Number of moles of gaseous products)-(Number of moles of
gaseous reactants)]
Kp = Kc (RT)∆n
5/10/2020 A.V.REDDY,RGUKT,RKVALLEY 9](https://image.slidesharecdn.com/chemicalequilibrium-231106060130-2a0d0b67/85/Chemical-Equilibrium-pptx-9-320.jpg)





![Factors affecting equilibrium
Lechateliers principle: When a system at equilibrium undergoes stress the equilibrium shifts in such a direction in
order to undo the stress
There are 3 type of stress:
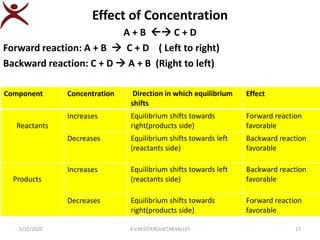
1) Changing concentration:
R P
At equilibrium,
If concentration of reactants increases()
equilibrium shifts towards products side(Right)
So that, [Reactants] decreases , [Products] increases
Similarly ,
If concentration of products increases ()
equilibrium shifts towards reactants side(left)
So that, [Reactants] increases , [Products] decreases
2) Changing pressure.
3) Changing Temperature.
5/10/2020 A.V.REDDY,RGUKT,RKVALLEY 15](https://image.slidesharecdn.com/chemicalequilibrium-231106060130-2a0d0b67/85/Chemical-Equilibrium-pptx-15-320.jpg)