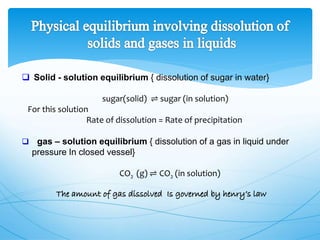
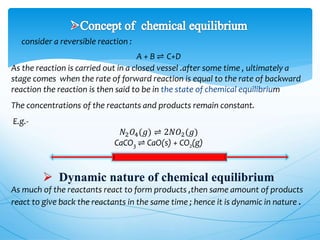
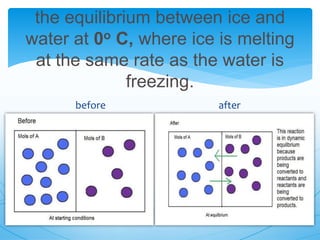
The document discusses chemical equilibrium. It defines equilibrium as a state where the rates of the forward and reverse reactions of a chemical process are equal, resulting in no net change in the concentrations or properties of the system. It provides examples of physical and chemical equilibrium processes. It describes key characteristics of equilibrium like dynamic nature, constant concentrations and temperatures, and the relationship between reaction rates and equilibrium constants.












![Law of mass action
“The rate at which a substance reacts is proportional to its active mass and
hence the rate of a chemical reaction is proportional to the product of the
active masses of the reaction”
Active mass = molar concentration OR it is the number of moles dissolved
per litre of solution
example: x g NaOH is dissolved in V liters of soln.
Then, active mass or molar concn
= 𝑥
40 moles in V liters
Consider a reaction A+B⟶products
Then
Rate at which A &B react together ∝ [A][B]
Or rate = k [A][B]](https://image.slidesharecdn.com/chemistrypptchemicalequilibrium-150123042256-conversion-gate02/85/Chemistryppt-chemical-equilibrium-16-320.jpg)

![consider a reversible reaction
A+B⇌C+D
thenAt equilibrium state,
Rate of forward reaction= kf [A][B] {kf is constant of proportionality}
Rate of backward reaction= kb [C][D] {kb is constant of proportionality}
Rate of forward reaction= Rate of backward reaction.
𝑘 𝑓 𝐴 𝐵 = 𝐾 𝐵 𝐶 𝐷
𝑘 𝑓
𝑘 𝑏
=
𝐶 𝐷
𝐴 𝐵
At constant temperature 𝑘 𝑓 and 𝑘 𝑏are constant. Therefore, substituting
𝑘 𝑓
𝑘 𝑏
by another
constant K we get,
𝐾 =
𝑘 𝑓
𝑘 𝑏
=
𝐶 𝐷
𝐴 𝐵
Here K is also a constant at constant temperature
And K is called EQUILIBRIUM CONSTANT](https://image.slidesharecdn.com/chemistrypptchemicalequilibrium-150123042256-conversion-gate02/85/Chemistryppt-chemical-equilibrium-18-320.jpg)

![General form
aA +bB⇌cC + dD
Kc =[C]c [D]d / [A]a [B]b
Where a, b, c and d have the same values as those in
the balanced chemical equation.
Chemical equation
aA + b B⇌c C + D
cC + d D⇌a A + b B
na A + nb B ⇌ncC + ndD
Equilibrium constant
K
K′c=(1/Kc)
K′″c= (Kcn)
Concentrations or partial pressure of pure solids or liquids do not appear
in the expression of the equilibrium constant.](https://image.slidesharecdn.com/chemistrypptchemicalequilibrium-150123042256-conversion-gate02/85/Chemistryppt-chemical-equilibrium-20-320.jpg)



![The equilibrium constant helps in finding the direction in which
reaction proceeds. For this, we have to calculate the reaction
quotient [Q].
The reaction quotient [Q] is the ratio of the product of
concentrations of the products to that of the reactants. For
example:
Consider the following reaction:
A + B ⇌ X + Y
The reaction quotient, 𝑄 = 𝑋 𝑌
𝐴 𝐵](https://image.slidesharecdn.com/chemistrypptchemicalequilibrium-150123042256-conversion-gate02/85/Chemistryppt-chemical-equilibrium-24-320.jpg)






