IB Chemistry Kinetics Design IA and uncertainty calculation for Order and Rate
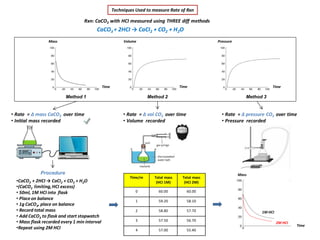
- 1. Techniques Used to measure Rate of Rxn Rxn: CaCO3 with HCI measured using THREE diff methods CaCO3 + 2HCI → CaCI2 + CO2 + H2O • Rate = Δ mass CaCO3 over time • Initial mass recorded •CaCO3 + 2HCI → CaCI2 + CO2 + H2O •(CaCO3 limiting,HCI excess) • 50ml, 1M HCI into flask • Place on balance • 1g CaCO3, place on balance • Record total mass • Add CaCO3 to flask and start stopwatch • Mass flask recorded every 1 min interval •Repeat using 2M HCI Method 1 Method 3Method 2 Mass Time Time Time Volume Pressure • Rate = Δ vol CO2 over time • Volume recorded • Rate = Δ pressure CO2 over time • Pressure recorded Procedure Time/m Total mass (HCI 1M) Total mass (HCI 2M) 0 60.00 60.00 1 59.20 58.10 2 58.80 57.70 3 57.50 56.70 4 57.00 55.40 Mass Time 2M HCI 1M HCI
- 2. Techniques Used to measure Rate of Rxn Rxn: CaCO3 with HCI measured using THREE diff methods CaCO3 + 2HCI → CaCI2 + CO2 + H2O • Rate = Δ mass CaCO3 over time • Initial mass recorded •CaCO3 + 2HCI → CaCI2 + CO2 + H2O •(CaCO3 limiting,HCI excess) • 50ml, 1M HCI into flask • Add 1g CaCO3 to flask and start stopwatch • Vol recordedevery 1 min interval •Repeat using 2M HCI Method 1 Method 3Method 2 Mass Time Time Time Volume Pressure • Rate = Δ vol CO2 over time • Volume recorded • Rate = Δ pressure CO2 over time • Pressure recorded Procedure Time/m Vol CO2 (HCI 1M) Vol CO2 (HCI 2M) 0 0.0 0.0 1 8.5 14.0 2 15.0 26.5 3 21.0 34.0 4 26.0 39.0 Volume CO2 Time 2M HCI 1M HCI
- 3. Techniques Used to measure Rate of Rxn Rxn: CaCO3 with HCI measured using THREE diff methods CaCO3 + 2HCI → CaCI2 + CO2 + H2O • Rate = Δ mass CaCO3 over time • Initial mass recorded •CaCO3 + 2HCI → CaCI2 + CO2 + H2O •(CaCO3 limiting,HCI excess) • 50ml, 1M HCI into flask • Add 1gCaCO3 to flask and start stopwatch • Press recorded every 1 min interval •Repeat using 2M HCI Method 1 Method 3Method 2 Mass Time Time Time Volume Pressure • Rate = Δ vol CO2 over time • Volume recorded • Rate = Δ pressure CO2 over time • Pressure recorded Procedure Time/m Pressure CO2 (HCI 1M) Pressure CO2 (HCI 2M) 0 101.3 101.3 1 102.4 103.4 2 103.5 105.6 3 110.3 115.2 4 113.5 118.2 Pressure CO2 Time 2M HCI 1M HCI
- 4. Techniques Used to measure Rate of Rxn • Rate = Δ mass Sulfur over time Method 1 Method 2 Mass Time Time Light Intensity • Rate = Δ light intensity over time • Light intensity recorded Procedure Conc/M S2O3 2- Time/s Rate 1/Time 0.2 80.8 1/80.8 = 0.0123 0.4 40.2 1/40.2 = 0.0248 0.6 25.2 1/25.2 = 0.0396 0.8 20.5 1/20.5 = 0.0487 1.0 18.2 1/18.2 = 0.0550 Rate = 1/time Conc Rxn: Na2S2O3 with HCI measured using TWO diff methods Na2S2O3 + 2HCI → 2NaCI2 + SO2 + H2O + S • Na2S2O3 + 2HCI → 2NaCI2 + SO2 + H2O + S • (Na2S2O3 limiting, HCI excess) •50ml 0.2M HCI into conical flask • Place on top of paper with cross X • Pour 5ml 0.1M Na2S2O3 into flask • Record time for X to disappear • Repeat with diff S2O3 2- conc Light sensor Light source 0.2 0.4 0.6 0.8
- 5. • Na2S2O3 + 2HCI → 2NaCI2 + SO2 + H2O + S • (Na2S2O3 limiting, HCI excess) •Pipette 1ml 0.2M S2O3 2- into cuvette • Pipette 0.1ml 0.1M HCI into cuvette • Mix and start light sensor • Record time for light intensity to drop • Repeat with diff S2O3 2- conc Techniques Used to measure Rate of Rxn • Rate = Δ mass Sulfur over time Method 1 Method 2 Mass Time Time Light Intensity • Rate = Δ light intensity over time • Light intensity recorded Procedure Conc/M S2O3 2- Time/s Rate 1/Time 0.2 80.8 1/80.8 = 0.0123 0.4 40.2 1/40.2 = 0.0248 0.6 25.2 1/25.2 = 0.0396 0.8 20.5 1/20.5 = 0.0487 1.0 18.2 1/18.2 = 0.0550 Rate = 1/time Rxn: Na2S2O3 with HCI measured using TWO diff methods Na2S2O3 + 2HCI → 2NaCI2 + SO2 + H2O + S Light source Light sensor Light intensity 0.8M S2O3 2- 1M S2O3 2- Conc 0.2 0.4 0.6 0.818.2 20.3 time
- 6. • H2O2 + 2KI + 2HCI → 2KCI + 2H2O + I2 (KIlimiting, H2O2 excess) • Pipette 5ml 3% H2O2, 5ml 0.1M HCI into flask • Add starch, 1ml 0.1M S2O3 to flask • Place on white paper with cross X • Pipette 5 ml 0.1M KI into flask • Record time for X to disappear • Repeat with diff KI conc Techniques Used to measure Rate of Rxn Method 1 Method 2 Mass iodine produced Time Time Absorbance • Rate = Δ Absorbance over time • Absorbance recorded Procedure Conc/M KI Time/s Rate 1/Time 0.00625 80.8 1/80.8 = 0.0123 0.0125 40.2 1/40.2 = 0.0248 0.025 25.2 1/25.2 = 0.0396 0.05 20.5 1/20.5 = 0.0487 0.1 18.2 1/18.2 = 0.0550 Rate = 1/time Conc Rxn: H2O2 with I - measured using TWO diff methods H2O2 + 2I- + 2H+ → 2H2O + I2 Iodine Clock Rxn H2O2 + 2I - + 2H+ → 2H2O + I2 I2 + 2S2O3 2- → S4O6 2- + 2I - I2 + starch → Blue black H2O2 - Oxidising Agent I - - Reducing Agent S203 2- - Reduce I2 to I – I2 - I2 react with starch form blue black • Rate = Δ mass iodine over time = Disappearance X due to blue black formation Abs increase when blue black form 0.025 0.05 0.1
- 7. • H2O2 + 2KI + 2HCI → 2KCI + 2H2O + I2 (KIlimiting, H2O2 excess) • Pipette 0.5ml 3% H2O2, 0.1M HCI to cuvette • Add starch, 0.1ml 0.1M S2O3 to cuvette • Pipette 0.5ml 0.2M KI to cuvette • Record Abs change • Repeat with diff KI conc Techniques Used to measure Rate of Rxn Method 1 Method 2 Mass iodine produced Time Time Absorbance • Rate = Δ Absorbance over time • Absorbance recorded Procedure Absorbance Time Abs increase when blue black form Rxn: H2O2 with I - measured using TWO diff methods H2O2 + 2I- + 2H+ → 2H2O + I2 Iodine Clock Rxn H2O2 + 2I - + 2H+ → 2H2O + I2 I2 + 2S2O3 2- → S4O6 2- + 2I - I2 + starch → Blue black H2O2 - Oxidising Agent I - - Reducing Agent S203 2- - Reduce I2 to I – I2 - I2 react with starch form blue black • Rate = Δ mass iodine over time = Disappearance X due to blue black formation Time Conc KI (0.2) Abs Conc KI (0.4) Abs Conc KI (0.6) Abs Conc KI (0.8) Abs 0 0.1 0.1 0.1 0.1 2 0.1 0.1 0.1 0.1 4 0.1 0.1 0.1 1.4 6 0.1 0.1 1.2 8 0.1 0.1 10 0.1 1.3 12 0.1 Rate 1/14 = 0.07 1/10 = 0.1 1/6 = 0.16 1/ 4 = 0.25 000000.2M KI0.8M KI 4 6 10 12
- 8. Techniques Used to measure Rate of Rxn Method 1 Method 2 Mass iodine produced Time Time Absorbance • Rate = Δ Absorbance over time • Absorbance recorded Procedure Conc KI /M Time/s Rate 1/Time 0.00625 80.8 1/80.8 = 0.0123 0.0125 40.2 1/40.2 = 0.0248 0.025 25.2 1/25.2 = 0.0396 0.05 20.5 1/20.5 = 0.0487 0.1 18.2 1/18.2 = 0.0550 Rate = 1/time Conc Rxn: S2O8 2- with I - measured using TWO diff methods Iodine Clock Rxn S2O8 2 - Oxidising Agent I - - Reducing Agent S203 2- - Reduce I2 to I – I2 - I2 react with starch form blue black • Rate = Δ mass iodine over time = Disappearance X due to blue black formation Abs increase when blue black form S2O8 2- + 2I - → 2SO4 2- + I2 S2O8 2- + 2I - → 2SO4 2- + I2 I2 + 2 S203 2- → S406 2- + 2I - I2 + starch → Blue black • S2O8 2- + 2I - → 2SO4 2- + I2 (KIlimiting, S2O8 2- excess) • Pipette 5ml 0.1M KI, 0.1M S2O3 • Add 1ml starch to flask • Place on white paper with cross X • Pipette 5 ml 0.1M S2O8 2- to flask • Record time for X to disappear • Repeat with diff KI conc 0.0125 0.025 0.05 0.1
- 9. • S2O8 2- + 2I - → 2SO4 2- + I2 (KIlimiting, S2O8 2- excess) • Pipette 0.5ml 0.1M KI, 0.1M S2O3 to cuvette • Add 0.1ml starch to cuvette • Pipette 0.5ml 0.1M S2O8 2- to cuvette • Record Abs change • Repeat with diff KI conc Techniques Used to measure Rate of Rxn Method 1 Method 2 Mass iodine produced Time Time Absorbance • Rate = Δ Absorbance over time • Absorbance recorded Procedure Rxn: S2O8 2- with I - measured using TWO diff methods Iodine Clock Rxn S2O8 2 - Oxidising Agent I - - Reducing Agent S203 2- - Reduce I2 to I – I2 - I2 react with starch form blue black • Rate = Δ mass iodine over time = Disappearance X due to blue black formation Abs increase when blue black form S2O8 2- + 2I - → 2SO4 2- + I2 S2O8 2- + 2I - → 2SO4 2- + I2 I2 + 2 S203 2- → S406 2- + 2I - I2 + starch → Blue black Time Conc KI (0.2) Abs Conc KI (0.4) Abs Conc KI (0.6) Abs Conc KI (0.8) Abs 0 0.1 0.1 0.1 0.1 2 0.1 0.1 0.1 0.1 4 0.1 0.1 0.1 1.4 6 0.1 0.1 1.2 8 0.1 0.1 10 0.1 1.3 12 0.1 Rate 1/14 = 0.07 1/10 = 0.1 1/6 = 0.16 1/ 4 = 0.25 Absorbance Time 0.8M KI 00000000000.2M KI 4 6 10 12
- 10. Techniques Used to measure Rate of Rxn Method 1 Method 2 Time Time Volume Pressure • Rate = Δ vol O2 over time • Volume recorded • Rate = Δ pressure O2 over time • Pressure recorded Procedure 2H2O2 → O2 + 2H2O Rxn: H2O2 with KI (catalyst)measured using TWO diff methods • 2H2O2 → O2 + 2H2O (H2O2 limiting,KI excess) • Pipette 1ml 1.0M KI to 20ml of 1.5% H2O2 • Vol O2 released recordedat 1 min interval • Repeated using 3% H2O2 conc Time/m Vol O2 (H2O2 1.5%) Vol O2 (H2O2 3.0%) 0 0.0 0.0 1 8.5 14.0 2 15.0 26.5 3 21.0 34.0 4 26.0 39.0 Volume O2 Time 3 % 1.5 %
- 11. • 2H2O2 → O2 + 2H2O (H2O2 limiting,KI excess) • Pipette 1ml 1.0M KI to 20ml of 1.5% H2O2 • Pressure O2 released recorded at 1 min interval • Repeat using 3% H2O2 conc Techniques Used to measure Rate of Rxn Method 1 Method 2 Time Time Volume Pressure • Rate = Δ vol O2 over time • Volume recorded • Rate = Δ pressure O2 over time • Pressure recorded Procedure 2H2O2 → O2 + 2H2O Time 3 % 1.5 % Time/m Pressure O2 (H2O2 1.5%) Pressure O2 (H2O2 3%) 0 101.3 101.3 1 102.4 103.4 2 103.5 105.6 3 110.3 115.2 4 113.5 118.2 Pressure O2 Rxn: H2O2 with KI (catalyst)measured using TWO diff methods
- 12. • Rate = Δ Conc I2 over time • Conc recorded using titration Techniques Used to measure Rate of Rxn Method 1 Method 2 Conc iodine produced Time Time Absorbance • Rate = Δ Absorbance over time • Absorbance recorded Procedure Absorbance Time Abs increase when iodine form 2Fe3+ + 2I - → 2Fe2+ + I2 Rxn: Fe3+ + I - measured using TWO diff methods Fe 3+ - Oxidising Agent I - - ReducingAgent • 2Fe3+ + 2I - → 2Fe2+ + I2 •(I - limiting, Fe3+ excess) • Pipette 1.5ml 0.02M Fe3+ to cuvette. • Find λ max for Fe3+ (450nm) • Abs vs time , select λ = 450nm • Pipette 1.0ml 0.02M KI to cuvette • Measure abs increase due to I2 formation • Repeat using diff KI conc Time/s Conc 0.02M KI Abs Conc 0.04M KI Abs 0 0.240 0.240 1 0.245 0.260 2 0.257 0.330 3 0.300 0.390 4 0.330 0.540 0.04 M 0.02 M
- 13. • 2Fe3+ + 2I - → 2Fe2+ + I2 (I - limiting, Fe3+ excess) • Pipette 25ml 0.02M KI /Fe3+ to flask. • Start time • Every 5min, pipette 10ml sol mix to flask • Titrate with S2O3 2- ( I2 form will react with S2O3 2- ) Amt I2 produced is determine. • I2 + 2S203 2- → S4O6 2- + 2I – (Mol ratio 1:2) • Rate = Δ Conc I2 over time • Conc recorded using titration Techniques Used to measure Rate of Rxn Method 1 Method 2 Conc iodine produced Time Time Absorbance • Rate = Δ Absorbance over time • Absorbance recorded Procedure Conc I2 Time 2Fe3+ + 2I - → 2Fe2+ + I2 Rxn: Fe3+ + I - measured using TWO diff methods Fe 3+ - Oxidising Agent I - - ReducingAgent Time/m Vol S2O3/ cm3 Conc I2/M 0 0 0 5 6 0.06 10 18 0.18 15 28 0.28 20 28 0.28 25 ml 0.02M KI/Fe3+ 10ml removed every 5m 0.2M S2O3 3- Contain I2 2S203 2- + I2 → S4O6 2- + 2I – 2 mol S203 2 – 1 mol I2 0.0012 mol – 0.006 mol I2 Vol S203 2- 6.0ml – Amt S203 2- = M x V = 0.2 x 0.006 = 0.0012 mol Conc I2 = Amt I2/Vol = 0.0006/0.01 = 0.06 M
- 14. • I2 + CH3COCH3 → CH3COCH2I + H+ + I – (CH3COCH3 limiting, I2 excess) • Pipette 1ml 0.002M I2 to cuvette. • Abs vs Time (λ max = 520nm) • Pipette 0.4ml 2M HCI and 1ml water to cuvette • Pipette 0.4ml 0.2M CH3COCH3 to cuvette • Record drop in abs over time • Repeat using diff CH3COCH3 conc • Rate = Δ Conc I2 over time • Conc recorded Techniques Used to measure Rate of Rxn Method 1 Method 2 Conc iodine Time Time Absorbance I2 • Rate = Δ Absorbance over time • Absorbance recorded Procedure Time Abs decrease I2 consumed I2 + CH3COCH3 → CH3COCH2I + H+ + I - Rxn: I2 + CH3COCH3 measured using TWO diff methods Time Conc (0.2M) Abs Conc (0.4M) Abs Conc (0.6M) Abs 0 2.00 2.00 2.00 2 1.86 1.76 1.52 4 1.75 1.54 1.20 6 1.57 1.24 0.78 8 1.23 1.23 0.56 10 1.10 0.78 0.40 Rate Gradient Time 0 Gradient Time 0 Gradient Time 0 Absorbance I2 0.2 M 0.4 M0.6 M Conc CH3COCH3 Rate
- 15. • Rate = Δ Conc I2 over time • Conc obtain from std calibrationplot Techniques Used to measure Rate of Rxn Method 1 Method 2 Conc iodine Time Time Absorbance I2 • Rate = Δ Absorbance over time • Absorbance recorded Procedure I2 + CH3COCH3 → CH3COCH2I + H+ + I - Rxn: I2 + CH3COCH3 measured using TWO diff methods Time Conc I2 (0.2M) Abs Conc I2 (0.4M) Abs Conc I2 (0.6M) Abs 0 2.00 2.00 2.00 2 1.86 1.76 1.52 4 1.75 1.54 1.20 6 1.57 1.24 0.78 Absorbance I2 0.2 M 0.4 M0.6 M Conc I2 • I2 + CH3COCH3 → CH3COCH2I + H+ + I – (CH3COCH3 limiting, I2 excess) • Pipette 1ml 0.002M I2 to cuvette. • Prepare std calibration plot Abs vs I2 conc • Abs vs Time (λ max = 520nm) • Pipette 0.4ml 2M HCI and 1ml water to cuvette • Pipette 0.4ml 0.2M CH3COCH3 to cuvette • Record drop in abs over time • Repeat using diff I2 conc Convert Abs I2 to conc I2 using std calibration curve Time 0.2 M 0.4 M0.6 M Conc I2 Abs 0 0 0.125 0.3 0.25 0.5 0.5 0.7 1.0 1.1 Std calibration curve Time
- 16. GraphicalRepresentationof Order :ZERO, FIRST and SECOND order ZERO ORDER FIRST ORDER SECOND ORDER Rate – 2nd order respect to [A] Conc x2 – Rate x 4 Unit for k Rate = k[A]2 Rate = kA2 k = M-1s-1 Rate Conc reactant Rate Conc reactant Conc reactant Conc Conc Conc Time Time Time Time Conc reactant Rate Time ln At Time 1/At ktAA ot ][][ Rate = k[A]0 Rate independent of [A] Unit for k Rate = k[A]0 Rate = k k = Ms-1 Rate vs Conc – Constant Conc vs Time – Linear Rate = k[A]1 Rate - 1st order respect to [A] Unit for k Rate = k[A]1 Rate = kA k = s-1 Rate vs Conc - proportional Conc vs Time ktAA eAA ot kt ot ]ln[]ln[ ][][ [A]t [A]o kt AA ot ][ 1 ][ 1 ln Ao 1/Ao Conc at time t Conc at time t
- 17. Using 2nd methodto find order Determinationorder: Na2S2O3 + 2HCI → NaCI + H2O + S + SO2 Order of Na2S2O3 Conc Na2S2O3 changes, fix [HCI] = 0.1M Na2S2O3 added HCI was added Time taken X fade away Conc Na2S2O3 Time/s Trial 1 ±0.01 Time/s Trial 2 ±0.01 Time/s Trial 3 ±0.01 Average time Rate 0.05 102.96 103.23 114.80 107.00 0.00046 0.10 45.43 44.08 38.35 42.62 0.0023 0.15 27.36 27.13 26.36 26.95 0.0055 0.20 18.06 18.57 17.53 18.05 0.0111 0.25 15.26 15.44 16.88 15.86 0.0158 Result expt 00046.0 107 05.0 . timeAve Conc Rate Cal for Conc 0.05M 4 ways for uncertainty rate 1st method Ave time = (107.00 ± 0.01) % uncertainty time = 9.34 x 10-3 % %∆ Rate = %∆ Time Rate = 0.00046 ± 9.34 x 10-3 % = 0.00046 ± 0.000000043 Too small Poor choice 4th method Uncertainty rate = (Max – min) for rate Rate 1 = Conc/time 1 = 0.05 / 102.96 = 0.00049 Rate 2 = Conc/time 2 = 0.05 / 103.23 = 0.00048 Rate 3 = Conc/ time 3 = 0.05 / 114.80 = 0.00043 Max rate = 0.00049 Min rate = 0.00043 Range = (Max – Min)/2 Range = (0.00049 – 0.00043)/2 = 0.00003 Average rate = (R1 + R2 + R3)/3 = 0.00047 ± 0.00003 Consistent Good choice 3rd method Uncertainty rate = std deviation (for conc 0.05) Rate 1 = Conc/time 1 = 0.05 / 102.96 = 0.00049 Rate 2 = Conc/time 2 = 0.05 / 103.23 = 0.00048 Rate 3 = Conc / time 3 = 0.05 / 114.80 = 0.00043 Average rate = (R1 + R2 + R3)/3 = 0.00047 ± std dev = 0.00047 ± 0.000032 Consistent Good choice 2nd method Using Range (Max – Min) for time Range = (Max – Min) for time/2 Range = (114.80 – 102.96)/2 = 5.92 Ave time = (107.00 ± 5.92) % uncertainty time = 5.5% % ∆Rate = %∆Time Rate = 0.00046 ± 5.5% = 0.00046 ± 0.000026 Consistent Good choice
- 18. Determinationorder : Na2S2O3 + 2HCI → NaCI + H2O + S + SO2 Order of Na2S2O3 Conc Na2S2O3 changes, fix [HCI] = 0.1M Na2S2O3 added HCI was added Time taken X fade away Conc Na2S2O3 Time/s Trial 1 ±0.01 Time/s Trial 2 ±0.01 Time/s Trial 3 ±0.01 Average time Rate 0.05 102.96 103.23 114.80 107.00 0.00046 0.10 45.43 44.08 38.35 42.62 0.0023 0.15 27.36 27.13 26.36 26.95 0.0055 0.20 18.06 18.57 17.53 18.05 0.0111 0.25 15.26 15.44 16.88 15.86 0.0158 Result expt 00046.0 00.107 05.0 . timeAve Conc Rate Cal for Conc 0.05M 2nd method Using Range (Max – Min) for time Range = (Max – Min)/2 Range = (114.80 – 102.96)/2 = 5.92 Ave time = (107.00 ± 5.92) % uncertainty time = 5.5% % ∆Rate = %∆Time Rate = 0.00046 ± 5.5% = 0.00046 ± 0.000026 Consistent Good choice Uncertaintyrate for conc 0.05M Conc Na2S2O3 Time/s Trial 1 ±0.01 Time/s Trial 2 ±0.01 Time/s Trial 3 ±0.01 Average time ± Time Range (Max- Min)/2 % ±Time Rate(±rate) 0.05 102.96 103.23 114.80 107.00 (114.8-102.96)/2= 5.92 5.5% 0.00046±0.000026 0.10 45.43 44.08 38.35 42.62 (45.43 – 38.35)/2 = 3.54 8.3% 0.0023 ±0.00027 0.15 27.36 27.13 26.36 26.95 (27.13 – 26.36)/2 = 0.50 1.8% 0.0055 ±0.00022 0.20 18.06 18.57 17.53 18.05 (18.06 – 17.53)/2 = 0.52 2.8% 0.0111 ±0.0006 0.25 15.26 15.44 16.88 15.86 (16.88 – 15.26)/2 = 0.81 5.1% 0.0158 ±0.0011
- 19. Determinationorder: Na2S2O3 + 2HCI → NaCI + H2O + S + SO2 Plot of Conc vs Rate Conc Na2S2O3 Rate(±rate) 0.05 0.00046±0.0000026 0.10 0.0023 ±0.00027 0.15 0.0055 ±0.00022 0.20 0.0111 ±0.0006 0.25 0.0158 ±0.0011 Order for Na2S2O3 (fix conc HCI) Let Rate = k[Na2S2O3]x [HCI] y Rate Conc Na2S2O3 Uncertainty rate Conc Na2S2O3 Rate Best fit Order = 2.21 Best fit Order = 2.21 Max fit Order = 2.29 Min fit Order = 2.12 Lowest uncertainty (Lowest Conc) to Highest uncertainty (Highest Conc) Highest uncertainty (Lowest Conc) to Lowest uncertainty (Highest Conc) Max order Min order
- 20. Determinationorder: Na2S2O3 + 2HCI → NaCI + H2O + S + SO2 Conc Na2S2O3 Rate(±rate) 0.05 0.00046±0.0000026 0.10 0.0023 ±0.00027 0.15 0.0055 ±0.00022 0.20 0.0111 ±0.0006 0.25 0.0158 ±0.0011 Conc Na2S2O3 Rate(±rate) 0.05 0.00044 0.10 0.00221 0.15 0.0055 0.20 0.0114 0.25 0.017 Max order Max fit Order = 2.29 Max order – Lowest uncertainty (Lowest Conc) to Highest uncertainty (Highest Conc) Conc Na2S2O3 Rate(±rate) 0.05 0.00046±0.0000026 0.10 0.0023 ±0.00027 0.15 0.0055 ±0.00022 0.20 0.0111 ±0.0006 0.25 0.0158 ±0.0011 Min order Conc Na2S2O3 Rate(±rate) 0.05 0.00048 0.10 0.00248 0.15 0.0055 0.20 0.0108 0.25 0.0147 Conc Na2S2O3 Conc Na2S2O3 Rate Rate Min fit Order = 2.12 Min order – Highest uncertainty (Lowest Conc) to Lowest uncertainty (Highest Conc) Highest uncertainty 0.0158 + 0.0011 = 0.017 Lowest uncertainty 0.00046 – 0.000026 = 0.00044 Highest uncertainty 0.00046 + 0.000026 = 0.00048 Lowest uncertainty 0.0158 – 0.0011 = 0.0147 Lowest uncertainty Highest uncertainty Lowest uncertainty Highest uncertainty Max order Min order
- 21. Order respect to Na2S2O3 = 2.21 Theoretical order = 2.00 % Error order = 10.7% Determinationorder: Na2S2O3 + 2HCI → NaCI + H2O + S + SO2 Conc Na2S2O3 Rate(±rate) 0.05 0.00046±0.0000026 0.10 0.0023 ±0.00027 0.15 0.0055 ±0.00022 0.20 0.0111 ±0.0006 0.25 0.0158 ±0.0011 Order for Na2S2O3 (fix conc HCI) Let Rate = k[Na2S2O3]x [HCI] 1 Order x = 2.21 Conc Na2S2O3 Rate Best fit Order = 2.21 Max fit Order = 2.29 Min fit Order = 2.12 Uncertainty order = (Max order – Min order)/2 %7.10%100 00.2 )00.221.2( ± Uncertaintyfor order = (Max – Min order)/2 Max order = 2.29 Min order = 2.12 ± Uncertaintyorder (Max – Min)/2 = ( 2.29 – 2.12)/2 = 0.09 ± Uncertaintyorder = 2.21 ± 0.09 % uncertainty order = (0.09/2.21)x 100 % = 4% % Error order = 10.7% % Uncertainty (Random Error) % Uncertainty (SystematicError) 4% % Error = % Random + % Systematic error error % Systematic = (10.7 – 4 )= 6.7% error Correct Method !
- 22. Order respect to Na2S2O3 = 2.21 Theoretical order = 2.00 % Error order = 10.7% Determinationorder: Na2S2O3 + 2HCI → NaCI + H2O + S + SO2 Conc Na2S2O3 Rate(±rate) 0.05 0.00046±0.0000026 0.10 0.0023 ±0.00027 0.15 0.0055 ±0.00022 0.20 0.0111 ±0.0006 0.25 0.0158 ±0.0011 Order for Na2S2O3 (fix conc HCI) Let Rate = k[Na2S2O3]x [HCI] 1 Order x = 2.21 Conc Na2S2O3 Rate Best fit Order = 2.21 % Uncertainty rate = % Uncertainty time = 5.5% %7.10%100 00.2 )00.221.2( % Error order = 10.7% % Uncertainty (Random Error) % Uncertainty (SystematicError) 5.5% Conc Na2S2O3 Time/s Trial 1 ±0.01 Time/s Trial 2 ±0.01 Time/s Trial 3 ±0.01 Average time ± Time Range (Max- Min)/2 % ±Time 0.05 102.96 103.23 114.80 107.00 (114.8-102.96)/2= 5.92 5.5% 0.10 45.43 44.08 38.35 42.62 (45.43 – 38.35)/2 = 3.54 8.3% 0.15 27.36 27.13 26.36 26.95 (27.13 – 26.36)/2 = 0.50 1.8% 0.20 18.06 18.57 17.53 18.05 (18.06 – 17.53)/2 = 0.52 2.8% 0.25 15.26 15.44 16.88 15.86 (16.88 – 15.26)/2 = 0.81 5.1% Wrong Method ! % Error = % Random + % Systematic error error % Systematic = (10.7 – 5.5)= 5.2 % error
