C5e Gas Volumes
•Download as DOC, PDF•
1 like•2,261 views
The document provides information about measuring gas volumes produced in chemical reactions, including: 1. Methods to measure gas volumes using apparatus like gas syringes and measuring cylinders. 2. Examples of reactions that produce gas volumes like Mg + 2HCl and CaCO3 + 2HCl. 3. Factors that determine gas volumes like the amount of limiting reactant and excess reactant.
Report
Share
Report
Share
Recommended
homeostasis questions.doc

The document contains questions and diagrams about homeostasis in the human body. It addresses the removal of waste such as water, carbon dioxide, urea and salts from the body. Specific organs involved in this process include the lungs, liver, kidneys and skin. The questions assess understanding of the role of these organs in maintaining homeostasis and water balance in the body.
3 - main idea ppt

The document discusses how to identify the main idea and supporting details of a text. It explains that the main idea is the most important part of a story or paragraph and tells what it is about. Supporting details describe and provide evidence for the main idea to make it stronger. Examples are provided of passages and questions are asked to help identify the main idea and supporting details.
CAPE Communication Studies IA

The document is a student portfolio on depression among teenagers in Trinidad and Tobago. It includes an introduction outlining the purpose of examining this issue and discussing the author's personal connection. It also includes a table of contents, preface, reflective short story, and analysis. The reflective short story, titled "Beena's Dilemma", depicts the life of a teenager named Beena who is suffering from depression due to bullying at school and abuse at home from her alcoholic father. After being verbally abused by her father one night, Beena attempts suicide by slashing her wrists. She is rushed to the hospital and survives. Her parents realize their role in her depression and seek counselling and therapy to help Beena
Communication Studies Internal Assessment SAMPLE

This Communication Studies IA sample is to be used as a guide to CAPE level (grade 12) students. The theme of this internal assessment is Social Media and Beauty.
Literary devices

The document defines and provides examples of several common literary devices:
- Hyperbole is exaggeration, like claiming to eat a ton of food.
- Personification gives human traits to non-human things, like saying curtains danced.
- Alliteration uses repeating sounds, like "sally sells seashells."
- Onomatopoeia imitates sounds in words, like "bow-wow" for a dog.
- A simile directly compares two things using like or as, while a metaphor implies a comparison.
- An oxymoron combines contradictory terms, such as "pretty ugly."
Osmosis, diffusion, active transport

The cherries lose their fleshy juicy texture because water moves out of the cherry cells into the hypertonic sugar solution by osmosis, causing the cherry cells to shrink and become plasmolysed.
2022 CAPE Regional Merit List

This document lists the top performing candidates from the Caribbean Examinations Council's (CEC) 2022 CAPE examinations by subject and territory. It provides the names, candidate numbers, grades, schools, and territories/countries of the top 10 candidates in various subjects including Accounting, Agricultural Science, Applied Mathematics, Art and Design, Biology, Caribbean Studies, Chemistry, and Communication Studies. The subjects, schools, territories/countries, ranks, grades, names, and candidate numbers of each top candidate are displayed in a table format.
Grade 8 biology first term 2013 copy

This document contains a biology test for grade 8 students with multiple choice and short answer questions. It tests knowledge of plant and animal cell structure and function, transport processes, photosynthesis, and enzyme action. The test has three sections - multiple choice questions, short answer definitions and explanations, and longer answer questions involving diagrams. Overall, the test examines key concepts in middle school biology.
Recommended
homeostasis questions.doc

The document contains questions and diagrams about homeostasis in the human body. It addresses the removal of waste such as water, carbon dioxide, urea and salts from the body. Specific organs involved in this process include the lungs, liver, kidneys and skin. The questions assess understanding of the role of these organs in maintaining homeostasis and water balance in the body.
3 - main idea ppt

The document discusses how to identify the main idea and supporting details of a text. It explains that the main idea is the most important part of a story or paragraph and tells what it is about. Supporting details describe and provide evidence for the main idea to make it stronger. Examples are provided of passages and questions are asked to help identify the main idea and supporting details.
CAPE Communication Studies IA

The document is a student portfolio on depression among teenagers in Trinidad and Tobago. It includes an introduction outlining the purpose of examining this issue and discussing the author's personal connection. It also includes a table of contents, preface, reflective short story, and analysis. The reflective short story, titled "Beena's Dilemma", depicts the life of a teenager named Beena who is suffering from depression due to bullying at school and abuse at home from her alcoholic father. After being verbally abused by her father one night, Beena attempts suicide by slashing her wrists. She is rushed to the hospital and survives. Her parents realize their role in her depression and seek counselling and therapy to help Beena
Communication Studies Internal Assessment SAMPLE

This Communication Studies IA sample is to be used as a guide to CAPE level (grade 12) students. The theme of this internal assessment is Social Media and Beauty.
Literary devices

The document defines and provides examples of several common literary devices:
- Hyperbole is exaggeration, like claiming to eat a ton of food.
- Personification gives human traits to non-human things, like saying curtains danced.
- Alliteration uses repeating sounds, like "sally sells seashells."
- Onomatopoeia imitates sounds in words, like "bow-wow" for a dog.
- A simile directly compares two things using like or as, while a metaphor implies a comparison.
- An oxymoron combines contradictory terms, such as "pretty ugly."
Osmosis, diffusion, active transport

The cherries lose their fleshy juicy texture because water moves out of the cherry cells into the hypertonic sugar solution by osmosis, causing the cherry cells to shrink and become plasmolysed.
2022 CAPE Regional Merit List

This document lists the top performing candidates from the Caribbean Examinations Council's (CEC) 2022 CAPE examinations by subject and territory. It provides the names, candidate numbers, grades, schools, and territories/countries of the top 10 candidates in various subjects including Accounting, Agricultural Science, Applied Mathematics, Art and Design, Biology, Caribbean Studies, Chemistry, and Communication Studies. The subjects, schools, territories/countries, ranks, grades, names, and candidate numbers of each top candidate are displayed in a table format.
Grade 8 biology first term 2013 copy

This document contains a biology test for grade 8 students with multiple choice and short answer questions. It tests knowledge of plant and animal cell structure and function, transport processes, photosynthesis, and enzyme action. The test has three sections - multiple choice questions, short answer definitions and explanations, and longer answer questions involving diagrams. Overall, the test examines key concepts in middle school biology.
CAPE Caribbean Studies Unit 2 - Sample SBA

CAPE Caribbean Studies Unit 2. This can provide guidelines as to what should be expected of your SBA.
Limericks

Limericks are short, humorous poems that have a strict rhyme scheme of AABBA, with lines 1, 2 and 5 rhyming together and lines 3 and 4 rhyming together. An example limerick is provided about a mouse running up a clock, as well as another about an old man with a beard. Instructions are given for writing your own limericks, including a partially completed example and a note that limericks can be set to song.
Implementing Science Investigations for the CSEC SBA

This document outlines a workshop for science teachers to be held on October 17-18, 2013. The workshop will be presented by Dr. Marcia Rainford and will focus on three key areas: planning and implementing science investigations for the CSEC exams, monitoring student progress as part of the school-based assessment process, and understanding suitable science investigations in physics, chemistry and biology. Teachers will also examine their responsibilities for student-based assessments and how to individualize instruction to meet student needs. The workshop will provide guidance on developing long-term plans for building students' investigative skills and selecting suitable investigation projects.
PPT Main Idea

The document provides guidance on identifying the main idea and supporting details of a text. It explains that every story or paragraph has a main idea, which is the most important part and tells what the story is about. Supporting details describe and provide examples for the main idea to make it stronger. Examples are provided of passages and questions are asked to identify the main idea and supporting details.
The Fox And The Crow

A crow had stolen some cheese and was sitting in a tree eating it. A fox saw the crow and wanted the cheese. The fox flattered the crow by calling her the Queen of Birds. When the crow opened her mouth to sing, the cheese fell to the ground. The fox then ate the cheese and told the crow not to trust flatterers. The story is about a crow that loses its cheese to a trickster fox because it falls for the fox's flattery.
Birdshooting season.pptx

The poem "Birdshooting Season" describes the rituals and activities that take place in a household in preparation for birdshooting season. The men give great care and attention to their guns, portrayed as "making marriages" with them. They gather at the narrator's house, imbuing it with a macho atmosphere. Meanwhile, the unhappy women stay up all night preparing food and drinks for the men. In the morning, the men depart in the dark with their guns and provisions, neglecting to acknowledge anyone else. The young boys wish to emulate the birdhunters, while the girls quietly encourage the birds to escape being shot.
Main idea slideshow

Use this main idea slideshow when introducing or reviewing main idea and supporting details with your 3rd, 4th, or 5th grade students. It helps students think about how titles are related to the main idea, the difference between main idea and details, and how details should support the main idea.
CAPE® 2021 Merit List by Subject from Caribbean Examinations Council

The document lists the top performing candidates from the Caribbean region in various CAPE subjects in the June 2021 examinations. It provides the names, schools, grades and countries of the top 10 candidates in subjects like Accounting, Agricultural Science, Applied Mathematics, Art and Design, Biology, Chemistry and others. The top candidate for Communication Studies was Angelina Jaya Siew from Naparima Girls' High School in Trinidad and Tobago.
How to create a paper boat

This document provides instructions for creating a paper boat using origami. It begins with an introduction to origami, explaining that it is the Japanese art of paper folding. It then lists reasons to learn origami, such as for survival kits, affordable toys/games, and making pocket money. The instructions section notes that you need one rectangular piece of paper to make the boat. It concludes by recapping that you can now create a paper boat in under 5 minutes using any foldable paper.
Eng sba

This document is Rollando Williams' English SBA on healthy lifestyles in Jamaica. It includes an acknowledgment, plan of investigation, three reflections on artifacts chosen, and plans for an oral presentation and group report. The artifacts are a song about healthy living, a poem titled "Health is Wealth", and a video on benefits of a healthy lifestyle. Through this SBA, Rollando aims to improve his English comprehension and research skills while learning about healthy living benefits.
Chemistry

This document provides a report on the performance of candidates who sat exams for the Caribbean Examinations Council's Advanced Proficiency Examination in Chemistry in May/June 2010. It summarizes the exam structure, number of candidates, and provides analysis of candidate performance on each exam paper and module. Overall, candidate performance was adequate, though some weaknesses were observed, particularly in calculations, writing equations, and practical skills. The report makes recommendations to teachers on strategies to help students improve.
Geogaphy sba

The major factors that contribute to tourism development in Ocho Rios, St. Ann include its location, physical features, and population. Ocho Rios benefits from its proximity to the airport and a cruise ship dock. Its beaches, mountains, and coastal landscape attract tourists. While some residents harass tourists, others contribute to tourism through small businesses showcasing Jamaican culture, food, and art. The physical environment and local population have both helped and hindered tourism growth in Ocho Rios.
IGCSE Biology Revision Quiz - Plant Physiology

This document contains a 63 question quiz about plant structure and function, including questions about photosynthesis, plant tissues, transport processes, reproduction, and growth. It tests knowledge of topics like plant responses to stimuli, the roles of hormones and mineral nutrients, methods of asexual reproduction, pollination, seed and fruit development, seed dispersal, and germination requirements. The quiz appears to be designed to comprehensively assess understanding of key concepts in plant biology.
Diffusion lab report

This lab report examines how water temperature affects the rate of diffusion. The student hypothesized that a higher water temperature would increase the rate of diffusion. Experimental results showed that diffusion occurred much faster in hot water compared to normal and cold water. For example, in one trial diffusion was completed in 186 seconds for hot water but took 600 seconds for normal water and 743 seconds for cold water. However, the student noted inconsistencies between trials that could be addressed in future experiments by standardizing testing conditions. Overall, the lab supported the hypothesis and provided insight into how temperature impacts diffusion, which is important for understanding cellular processes.
Communication studies i.a mikado (child abuse)

This document contains Mikado Meikle's internal assessment project on child abuse. It includes an acknowledgement, introduction, research questions, questionnaire, preface, and reflective piece. The introduction provides an overview of the project structure which will examine causes of child abuse and ways to lower its effects. It will have an expository and reflective section. The research questions explore the main causes of child abuse, influences on abusive acts, and strategies to decrease child abuse impacts. The reflective piece is a story titled "No Daddy" about a teenage girl experiencing physical and sexual abuse from her step-father.
Com stud

The document provides information about a student's internal assessment project on the impact of alcohol consumption among Jamaican teens. It includes an introduction outlining the topic, sources that will be used (a newspaper article, website, and interview), and the reflective piece titled "Pour it Out" describing a teen's experience with alcohol abuse. The reflective piece is analyzed focusing on the language variations used and communicative behaviors presented. The student acknowledges those who helped with the project, and provides a bibliography of sources.
Circulation in plants and animals

Internal transport in animals occurs through circulatory systems. Simple organisms rely on diffusion through their gastrovascular cavities or two cell layers. More complex animals have developed muscular pumps (hearts) that circulate fluid (blood or hemolymph) through tubular vessels. Most animals have closed circulatory systems with double circulation, where blood is pumped from the heart to the lungs and then to the rest of the body. The human cardiovascular system has further evolved to include arteries, veins, and a four-chambered heart that fully separates oxygenated and deoxygenated blood.
CAPE Accounting Internal Assessment SAMPLE

This is to be used as a guide for CAPE level Accounting students. The focus of this IA was the transition from manual to software accounting.
Theme: What It Is and How to Find It

This document discusses what a theme is and how to identify the theme of a story. It explains that a theme is the author's opinion on a subject conveyed through the story, not a subject itself. It provides examples of themes from well-known stories and gives tips for finding themes by examining how the main character changes, looking for direct statements of theme, analyzing the title, and considering the main conflict. The document emphasizes that stories can have multiple themes and there is no single right answer, as long as a claimed theme is supported by evidence from the text.
Social studies sba about domestic violence

this sba is about domestic violence you can view and download it for further usage my recommendation needs to be fixed i don't think it's accurate.
Space

The document discusses several topics related to astronomy and space, including:
- The formation of elements like hydrogen in the Big Bang and later fusion in stars that led to heavier elements like carbon.
- How satellites orbit Earth due to centripetal force balancing gravitational attraction, with examples of low polar orbits and geostationary orbits.
- How planets orbit the Sun due to centripetal force balancing gravitational attraction.
- How the Sun will evolve over time from its current stable phase into a red giant and later a white dwarf or neutron star/black hole.
Food chains project

The document defines key terms related to food chains and ecosystems:
1) Biomass refers to dead plant and animal material that can be used as fuel.
2) A food chain describes a series of living things connected by predation, with each organism eating the one below it.
3) Producers are plants that use sunlight to produce their own food.
4) Consumers are herbivores, zooplankton and carnivores that eat other organisms.
5) Herbivores eat only plants, carnivores eat meat, and predators hunt other animals for food.
More Related Content
What's hot
CAPE Caribbean Studies Unit 2 - Sample SBA

CAPE Caribbean Studies Unit 2. This can provide guidelines as to what should be expected of your SBA.
Limericks

Limericks are short, humorous poems that have a strict rhyme scheme of AABBA, with lines 1, 2 and 5 rhyming together and lines 3 and 4 rhyming together. An example limerick is provided about a mouse running up a clock, as well as another about an old man with a beard. Instructions are given for writing your own limericks, including a partially completed example and a note that limericks can be set to song.
Implementing Science Investigations for the CSEC SBA

This document outlines a workshop for science teachers to be held on October 17-18, 2013. The workshop will be presented by Dr. Marcia Rainford and will focus on three key areas: planning and implementing science investigations for the CSEC exams, monitoring student progress as part of the school-based assessment process, and understanding suitable science investigations in physics, chemistry and biology. Teachers will also examine their responsibilities for student-based assessments and how to individualize instruction to meet student needs. The workshop will provide guidance on developing long-term plans for building students' investigative skills and selecting suitable investigation projects.
PPT Main Idea

The document provides guidance on identifying the main idea and supporting details of a text. It explains that every story or paragraph has a main idea, which is the most important part and tells what the story is about. Supporting details describe and provide examples for the main idea to make it stronger. Examples are provided of passages and questions are asked to identify the main idea and supporting details.
The Fox And The Crow

A crow had stolen some cheese and was sitting in a tree eating it. A fox saw the crow and wanted the cheese. The fox flattered the crow by calling her the Queen of Birds. When the crow opened her mouth to sing, the cheese fell to the ground. The fox then ate the cheese and told the crow not to trust flatterers. The story is about a crow that loses its cheese to a trickster fox because it falls for the fox's flattery.
Birdshooting season.pptx

The poem "Birdshooting Season" describes the rituals and activities that take place in a household in preparation for birdshooting season. The men give great care and attention to their guns, portrayed as "making marriages" with them. They gather at the narrator's house, imbuing it with a macho atmosphere. Meanwhile, the unhappy women stay up all night preparing food and drinks for the men. In the morning, the men depart in the dark with their guns and provisions, neglecting to acknowledge anyone else. The young boys wish to emulate the birdhunters, while the girls quietly encourage the birds to escape being shot.
Main idea slideshow

Use this main idea slideshow when introducing or reviewing main idea and supporting details with your 3rd, 4th, or 5th grade students. It helps students think about how titles are related to the main idea, the difference between main idea and details, and how details should support the main idea.
CAPE® 2021 Merit List by Subject from Caribbean Examinations Council

The document lists the top performing candidates from the Caribbean region in various CAPE subjects in the June 2021 examinations. It provides the names, schools, grades and countries of the top 10 candidates in subjects like Accounting, Agricultural Science, Applied Mathematics, Art and Design, Biology, Chemistry and others. The top candidate for Communication Studies was Angelina Jaya Siew from Naparima Girls' High School in Trinidad and Tobago.
How to create a paper boat

This document provides instructions for creating a paper boat using origami. It begins with an introduction to origami, explaining that it is the Japanese art of paper folding. It then lists reasons to learn origami, such as for survival kits, affordable toys/games, and making pocket money. The instructions section notes that you need one rectangular piece of paper to make the boat. It concludes by recapping that you can now create a paper boat in under 5 minutes using any foldable paper.
Eng sba

This document is Rollando Williams' English SBA on healthy lifestyles in Jamaica. It includes an acknowledgment, plan of investigation, three reflections on artifacts chosen, and plans for an oral presentation and group report. The artifacts are a song about healthy living, a poem titled "Health is Wealth", and a video on benefits of a healthy lifestyle. Through this SBA, Rollando aims to improve his English comprehension and research skills while learning about healthy living benefits.
Chemistry

This document provides a report on the performance of candidates who sat exams for the Caribbean Examinations Council's Advanced Proficiency Examination in Chemistry in May/June 2010. It summarizes the exam structure, number of candidates, and provides analysis of candidate performance on each exam paper and module. Overall, candidate performance was adequate, though some weaknesses were observed, particularly in calculations, writing equations, and practical skills. The report makes recommendations to teachers on strategies to help students improve.
Geogaphy sba

The major factors that contribute to tourism development in Ocho Rios, St. Ann include its location, physical features, and population. Ocho Rios benefits from its proximity to the airport and a cruise ship dock. Its beaches, mountains, and coastal landscape attract tourists. While some residents harass tourists, others contribute to tourism through small businesses showcasing Jamaican culture, food, and art. The physical environment and local population have both helped and hindered tourism growth in Ocho Rios.
IGCSE Biology Revision Quiz - Plant Physiology

This document contains a 63 question quiz about plant structure and function, including questions about photosynthesis, plant tissues, transport processes, reproduction, and growth. It tests knowledge of topics like plant responses to stimuli, the roles of hormones and mineral nutrients, methods of asexual reproduction, pollination, seed and fruit development, seed dispersal, and germination requirements. The quiz appears to be designed to comprehensively assess understanding of key concepts in plant biology.
Diffusion lab report

This lab report examines how water temperature affects the rate of diffusion. The student hypothesized that a higher water temperature would increase the rate of diffusion. Experimental results showed that diffusion occurred much faster in hot water compared to normal and cold water. For example, in one trial diffusion was completed in 186 seconds for hot water but took 600 seconds for normal water and 743 seconds for cold water. However, the student noted inconsistencies between trials that could be addressed in future experiments by standardizing testing conditions. Overall, the lab supported the hypothesis and provided insight into how temperature impacts diffusion, which is important for understanding cellular processes.
Communication studies i.a mikado (child abuse)

This document contains Mikado Meikle's internal assessment project on child abuse. It includes an acknowledgement, introduction, research questions, questionnaire, preface, and reflective piece. The introduction provides an overview of the project structure which will examine causes of child abuse and ways to lower its effects. It will have an expository and reflective section. The research questions explore the main causes of child abuse, influences on abusive acts, and strategies to decrease child abuse impacts. The reflective piece is a story titled "No Daddy" about a teenage girl experiencing physical and sexual abuse from her step-father.
Com stud

The document provides information about a student's internal assessment project on the impact of alcohol consumption among Jamaican teens. It includes an introduction outlining the topic, sources that will be used (a newspaper article, website, and interview), and the reflective piece titled "Pour it Out" describing a teen's experience with alcohol abuse. The reflective piece is analyzed focusing on the language variations used and communicative behaviors presented. The student acknowledges those who helped with the project, and provides a bibliography of sources.
Circulation in plants and animals

Internal transport in animals occurs through circulatory systems. Simple organisms rely on diffusion through their gastrovascular cavities or two cell layers. More complex animals have developed muscular pumps (hearts) that circulate fluid (blood or hemolymph) through tubular vessels. Most animals have closed circulatory systems with double circulation, where blood is pumped from the heart to the lungs and then to the rest of the body. The human cardiovascular system has further evolved to include arteries, veins, and a four-chambered heart that fully separates oxygenated and deoxygenated blood.
CAPE Accounting Internal Assessment SAMPLE

This is to be used as a guide for CAPE level Accounting students. The focus of this IA was the transition from manual to software accounting.
Theme: What It Is and How to Find It

This document discusses what a theme is and how to identify the theme of a story. It explains that a theme is the author's opinion on a subject conveyed through the story, not a subject itself. It provides examples of themes from well-known stories and gives tips for finding themes by examining how the main character changes, looking for direct statements of theme, analyzing the title, and considering the main conflict. The document emphasizes that stories can have multiple themes and there is no single right answer, as long as a claimed theme is supported by evidence from the text.
Social studies sba about domestic violence

this sba is about domestic violence you can view and download it for further usage my recommendation needs to be fixed i don't think it's accurate.
What's hot (20)
Implementing Science Investigations for the CSEC SBA

Implementing Science Investigations for the CSEC SBA
CAPE® 2021 Merit List by Subject from Caribbean Examinations Council

CAPE® 2021 Merit List by Subject from Caribbean Examinations Council
Viewers also liked
Space

The document discusses several topics related to astronomy and space, including:
- The formation of elements like hydrogen in the Big Bang and later fusion in stars that led to heavier elements like carbon.
- How satellites orbit Earth due to centripetal force balancing gravitational attraction, with examples of low polar orbits and geostationary orbits.
- How planets orbit the Sun due to centripetal force balancing gravitational attraction.
- How the Sun will evolve over time from its current stable phase into a red giant and later a white dwarf or neutron star/black hole.
Food chains project

The document defines key terms related to food chains and ecosystems:
1) Biomass refers to dead plant and animal material that can be used as fuel.
2) A food chain describes a series of living things connected by predation, with each organism eating the one below it.
3) Producers are plants that use sunlight to produce their own food.
4) Consumers are herbivores, zooplankton and carnivores that eat other organisms.
5) Herbivores eat only plants, carnivores eat meat, and predators hunt other animals for food.
Ed and Matt

Photography derives from the Greek words for "light" and "to write" and the word was coined by Sir John Herschel in 1839. Early philosophers and mathematicians described the principles of the pinhole camera as early as the 5th century BC, though the first photographs were not made until combining several technical discoveries. Cameras are now widely used around the world to capture wonderful sights and are symbols of both paparazzi and human freedom and ingenuity.
Radiation

In alpha decay, the nucleus loses 2 protons and 2 neutrons, decreasing the atomic number by 2 and mass number by 4. In beta decay, a neutron changes to a proton and an electron is ejected, increasing the atomic number by 1 while keeping the mass number the same. In gamma decay, only energy is emitted with no change to the atomic or mass number.
The handy science answer book (the handy answer book series)

A nanometer is an incredibly small unit of measurement. Here are some comparisons to help illustrate just how small a nanometer is:
- A single gold atom is about a third of a nanometer in diameter.
- A strand of DNA is approximately 2-3 nanometers wide.
- A single-walled carbon nanotube, one of the thinnest nanomaterials, is about 1 nanometer in diameter.
- A sheet of paper is around 100,000 nanometers thick.
- A human hair is around 80,000-100,000 nanometers wide.
So in summary, a nanometer is about the size of several atoms lined up in a row, or the width of just a
Rate of reaction

The document discusses the rate of reaction and factors that affect it. It defines the rate of reaction as the change in amount of reactant or product over time. It provides examples of suitable measurable changes that can be used to calculate rate such as concentration, mass, volume of gas, and temperature. It also discusses different ways to measure the rate of reaction including finding the average rate over a time period and instantaneous rate by taking the gradient of the graph at a given time. Finally, it discusses several factors that can affect the rate of reaction such as surface area, temperature, concentration, catalyst, and pressure.
Factors affecting the rate of a chemical reaction

The document discusses factors that affect the rate of a chemical reaction, including concentration of reactants, temperature, surface area, and presence of catalysts. It describes experiments conducted to study the effect of these factors on the reaction of magnesium with hydrochloric acid, decomposition of hydrogen peroxide, and the reaction of iron with copper nitrate. The reaction rates were found to increase with increasing concentration and temperature, and increasing the surface area of solid reactants. A catalyst was also found to increase the rate of decomposition of hydrogen peroxide.
Viewers also liked (7)
The handy science answer book (the handy answer book series)

The handy science answer book (the handy answer book series)
Similar to C5e Gas Volumes
Rates of reaction

Here are the effects on the rate of reaction of changing each condition:
- If the concentration of acid is increased, the rate of reaction will increase. This is because there will be more reactant particles per unit volume, resulting in more frequent collisions between reactants.
- If calcium carbonate powder is used instead of small pieces, the rate of reaction will increase. This is because powder has a larger surface area, allowing for more reactant particles at the interface where the reaction occurs.
- If the volume of acid is increased, the rate of reaction will initially increase but then level off. This is because there will be more reactant particles initially, but the reaction will use them up over time.
- If the
Rate of reaction.pptx

1) Several factors that affect the rate of chemical reactions are discussed, including the size of reactants, concentration of solutions, temperature, and presence of catalysts.
2) When the size of reactants is smaller, the total surface area exposed to collisions increases, leading to a higher rate of reaction.
3) A higher concentration of reactants means more particles per unit volume, resulting in more frequent collisions and a faster rate.
4) At higher temperatures, reactant particles have greater kinetic energy to overcome the activation energy barrier, increasing the frequency of effective collisions and accelerating the rate.
5) Catalysts provide an alternative reaction pathway requiring lower activation energy, allowing more particles to react effectively and speed
Chapter 1

The document discusses the rate of chemical reactions and factors that affect it. It provides examples of reactions that occur at different rates and how rate is calculated. The average rate and instantaneous rate are defined. Experiments are described to determine the effect of surface area, concentration, temperature, catalyst, and pressure on the reaction rate. The concept of effective collision is introduced, where particles must collide with sufficient energy and correct orientation for a reaction to occur. Factors that increase collision frequency or lower activation energy can increase the reaction rate.
Uncertainty calculation for rate of reaction

This document describes experiments conducted to determine the kinetics and reaction orders of iodine clock and sulfur clock reactions. For the iodine clock reaction, the effect of changing the concentration of reactants on the reaction rate was examined. For the sulfur clock reaction, different methods for calculating uncertainty in rate measurements were compared. The activation energy of the iodine clock reaction was also calculated by measuring rates at different temperatures. Finally, the order of the iodine-propanone reaction was investigated by varying the concentrations of iodine, propanone and acid, and measuring changes in absorbance over time.
Reaction rates (Examville.com)

The document defines rate of reaction as the change in amount of reactants or products over time. It provides examples of fast and slow reactions. Rate can be determined by measuring decreases in reactants or increases in products over time. For reactions where quantities are immeasurable, rate is determined by time for a measurable change like color change. Graphs of quantity over time can be used to determine average rate from the slope or instantaneous rate from the tangent slope at a point. Examples show calculating rates from graphs, tables, and chemical equations.
Rate of reaction diagnostic

The document discusses the key concepts related to the rate of a chemical reaction:
1. The rate of reaction is affected by factors like concentration, temperature, surface area, and presence of a catalyst. A catalyst increases the rate by lowering the activation energy without being consumed in the reaction.
2. Collision theory states that molecules must collide with sufficient energy, called the activation energy, for a reaction to take place. Increasing effective collisions through factors like concentration and temperature increases the rate.
3. A catalyst facilitates the reaction by providing an alternative reaction pathway with lower activation energy, allowing more particles to react per unit time and increasing the rate.
Chapter 10 Rate of reaction

1. The document discusses factors that affect the rate of chemical reactions, including temperature, concentration, and the presence of catalysts.
2. One experiment showed that increasing the temperature of a sodium thiosulfate solution decreased the time for a chemical reaction, showing the rate of reaction increases with temperature.
3. Another experiment demonstrated that adding manganese (IV) oxide as a catalyst increased the rate of decomposition of hydrogen peroxide by allowing a glowing splint to relight.
4. In general, the document explains that collision theory states increasing temperature, concentration, or the use of catalysts increases the frequency and effectiveness of particle collisions, thus speeding up chemical reactions.
Enthalpy of formation of magnesium oxide

This document describes an experiment to determine the enthalpy of formation of magnesium oxide (MgO) through calorimetry. Three chemical reactions are used: 1) Mg + 2HCl → MgCl2 + H2; 2) MgO + 2HCl → MgCl2 + H2; 3) H2 + 1/2O2 → H2O. The enthalpy of formation of MgO is calculated by adding the enthalpies of reactions 1 and 3 and subtracting the enthalpy of reaction 2. Data on temperature changes is collected when Mg and MgO are added to HCl solution. The enthalpy of formation is found to be 590 kJ/mol,
Rate of reaction(f5)

A reaction can be either fast or slow depending on the time taken. A fast reaction has a short time and high rate, while a slow reaction has a long time and low rate. The rate of reaction depends on factors like concentration, temperature, surface area, and catalysts. Collisions between reactant particles must be effective, with sufficient energy to overcome the activation energy barrier, in order for reactions to occur.
Science example

The document summarizes a chemistry experiment that investigated how the concentration of hydrochloric acid (HCl) affects the rate of reaction when magnesium (Mg) is added. It was hypothesized that higher HCl concentrations would increase the reaction rate. Experiments were conducted using different HCl concentrations from 0.1M to 0.5M. The results showed that higher concentrations produced hydrogen gas faster, supporting the hypothesis. A graph of the reaction rates demonstrated they increased quadratically with increasing HCl concentration. However, the conclusion noted some weaknesses in temperature control and material reuse that could be improved.
Factors affecting rate of reaction (recovered)

The document discusses how several factors affect the rate of chemical reactions according to collision theory:
1. Increasing the surface area of reactants by decreasing their size increases the rate of reaction by increasing collision frequency.
2. Higher concentrations increase collision frequency by providing more particles in a given volume, likewise increasing the reaction rate.
3. Higher temperatures cause reactants to move faster and collide more frequently, also increasing the reaction rate.
4. Catalysts can lower the activation energy for a reaction, increasing the frequency of effective collisions and thereby accelerating the reaction.
5. For gas reactions, higher pressures compress the gas, providing more particles per volume and thus more collisions and a faster reaction.
Chapter 1 Rate of Reactions 

The document discusses rate of reaction and factors that affect it. It defines rate of reaction as the change in amount of reactants or products per unit time. It describes several factors that affect rate based on collision theory, including surface area, concentration, temperature, catalysts, and pressure. It gives examples of how scientific understanding of rate of reaction enhances quality of life, such as refrigeration, pressure cooking, cutting food into smaller pieces, making margarine, and burning coal.
SPM F5 Chapter 1 Rate of Reaction 

The document discusses rate of reaction and factors that affect it. It defines rate of reaction as the change in amount of reactants or products per unit time. Rate of reaction is affected by several factors including surface area, concentration, temperature, catalysts and pressure (for gas reactions). The collision theory is also explained, stating that reactions only occur during effective collisions where particles attain sufficient kinetic energy to overcome the activation energy barrier. Examples of how scientific understanding of rate of reaction enhances quality of life through applications like food storage, cooking and petroleum processing are provided.
Rates of reaction (1).pdf

The document discusses the rate of reaction and factors that affect it. It defines rate of reaction as the change in amount of reactant or product per unit time. The rate depends on concentration, temperature, surface area, and other factors. Higher concentrations and temperatures increase the rate by increasing collisions between reactant particles. Smaller particle sizes also increase the rate by providing more surface area for reactions. Graphs and experiments are described to illustrate these relationships.
Catalyst on-the-rate-of-reaction-teacher’s-guide

The document discusses how the amount of catalyst affects the rate of reaction. It describes two experiments:
1) Using 0.2g of manganese (IV) oxide powder as the catalyst.
2) Using 0.8g of manganese (IV) oxide powder as the catalyst.
The results show that with more catalyst (0.8g), the rate of reaction is faster as shown by the higher volume of oxygen gas liberated over time. Increasing the amount of catalyst increases the rate of reaction.
rate of rxns.pptx

The document discusses the rate of reaction and factors that affect it. It defines rate of reaction as the change in amount of reactants or products per unit time. The rate depends on several factors:
1) The surface area of solid reactants - Increasing the surface area by reducing particle size increases the rate, as there are more contact points for collisions.
2) Concentration of reactants - Higher concentrations increase the chance of collisions between reactant particles, speeding up the rate.
3) Temperature - Raising the temperature increases the kinetic energy of particles, making successful collisions that overcome the activation energy barrier for reaction more likely.
4) Pressure for gas reactions - Higher pressures increase the rate by squeezing
CHE451- CO2 Absorption Oral Report

The document summarizes an experiment studying factors that affect CO2 absorption in a NaOH solution. The experiment tested how absorption is affected by flow rate of CO2 gas, CO2 concentration, volume of NaOH solution, and pH. Absorption increased with lower flow rates, higher NaOH volumes, and higher pH. The results supported theories that more CO2 absorbs at higher pressures and NaOH concentrations. Future studies could optimize flow rates to maximize both absorption capacity and breakthrough time.
ScienceShare.co.uk Shared Resource

This experiment investigates how the size of calcium carbonate chips affects the rate of reaction with hydrochloric acid. Three different sized chips - large, medium, and small crushed chips - are reacted with a constant volume of hydrochloric acid, and the number of bubbles produced in the first minute is counted to measure the rate of reaction. The independent variable is the size of the calcium carbonate chips, the dependent variable is the rate of reaction measured by bubbles produced, and the constant variables include the volume of hydrochloric acid and reaction time of one minute.
Chem 162 Lab 3 Gas Laws Pa.docx

Chem 162 Lab 3: Gas Laws Part I & II- Sample Data for the class
1) Sample Data Group 1:
Part I
Part II
Volume (ml)
Pressure (kPa)
Temperature (°C)
Pressure (kPa)
103.0
60
70.8
113.5
88.0
70
66.3
112.6
73.0
85
61.8
111.5
62.0
100
57.1
110.4
44.0
140
51.5
109.0
34.0
180
39.9
105.5
31.0
200
26.4
101.8
10.5
96.7
2) Sample Data Group 2:
Part I
Part II
Volume (ml)
Pressure (Torr)
Temperature (°C)
Pressure (kPa)
32.0
630
57
109.6
29.2
690
52
108.4
27.8
726
48.5
107.4
25.6
790
43.6
106.3
24.2
843
38.1
104.8
22.2
914
33.1
103.5
29.3
102.2
25.4
101.1
22.5
100.1
20
99.4
17.4
98.6
12.8
97.2
9.4
96.7
Bellevue College | Chemistry 162
1
Empirical Gas Laws (Part 3): The Ideal Gas Law
Determination of the Universal Gas Constant, R
In this experiment, you will generate and collect a sample of hydrogen gas over water by the
reaction of magnesium with hydrochloric acid.
Using the Ideal Gas Law (PV=nRT) you will find values for the pressure (P), volume (V),
number of moles of the gas (n), and the temperature (T) in order to determine the gas constant
(R). Because there will be water vapor present in your sample, you will make a correction to the
measured pressure and then compare your result for R to the literature value.
In this experiment, you will:
Determine a value for the Universal Gas Constant, R. (Part 3 of Empirical Gas Laws)
Safety Precautions
Wear your goggles at all times. Hydrochloric acid is corrosive.
Avoid spills and contact with your skin and clothing. If HCl
comes in contact with your skin, inform your teacher and flush
the acid with large quantities of water.
Note: If you are doing Part 3 to determine the value of the Universal
Gas Constant, R in the same period as Parts 1 and 2, you should get Part 3
started first.
EXPERIMENTAL PROCEDURE (WORK IN PAIRS)
1. Put on goggles. Keep them on during the entire experiment.
2. Obtain a piece of magnesium ribbon that weighs no more than 0.08 grams. Record the mass
obtained (use significant figures!). Record this value in your data table (see report sheets).
Loosely roll it into a ball or coil it.
Encase the magnesium in a piece of copper mesh. Why do you think this might be helpful?
3. Fill the 800-mL beaker with approximately 200-mL of tap water.
4. Fill the 100-mL graduated cylinder with tap water. Using parafilm, a one-
hole stopper, or the palm of your hand, cover the top and invert the cylinder
into the beaker of water. You will end up with an inverted cylinder full of
water. Remove the parafilm or stopper if you used one. Rest the cylinder
on the bottom of the beaker. Try not to introduce any air bubbles in your
inverted cylinder (see Figure 1).
5. Place the magnesium (in its copper cage) into the graduated cylinder. Make
sure the magnesium is captured in the cylinder.
Figure 1: Gas collection in an
inverted cylinder full of water.
Relaciones estequiometricas.pptx

The document discusses stoichiometric relationships and reacting masses and volumes. It provides examples of mole to mole, mole to mass, mass to mole, and mass to mass stoichiometry problems. It also covers limiting reactants, theoretical yield, experimental yield, and calculating percent yield. The key ideas are that mole ratios can be used to calculate reacting quantities, the limiting reactant determines the theoretical yield, the experimental yield may differ from the theoretical yield, and percent yield compares the experimental to the theoretical yield.
Similar to C5e Gas Volumes (20)
More from M F Ebden
Motion

Forces add up as vectors, so a 10N force to the left and an 8N force to the right result in a 2N force to the left. If the net force is zero, the object will stay at rest or move at a constant velocity. If there is a net force, the object will accelerate according to F=ma. Projectiles follow parabolic paths and take the same time to fall as dropped objects. Kinetic energy equals half the mass times the velocity squared.
Light

The document summarizes key aspects of different parts of the electromagnetic spectrum including radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma rays. It also discusses properties that apply across the spectrum such as all waves traveling at the speed of light, interference and diffraction effects. Additionally, it covers topics in waves and optics including reflection, refraction, lenses, sound waves, and seismic waves.
Energy

Heat is transferred through particles vibrating (conduction), air rising when hot (convection), and radiation being absorbed and reflected. Insulation in walls traps air which is an insulator, while different methods like passive solar heating and fossil fuels generate heat energy through various processes. When a solid melts it changes state at a constant temperature.
Electomagnets

1) When a current passes through a copper wire in a magnetic field, it induces a voltage in the wire due to electromagnetic induction.
2) An alternating current generator uses a rotating coil in a magnetic field to generate a current, where the direction of the current reverses as the coil rotates.
3) Transformers use the principle of electromagnetic induction to change the voltage of an alternating current - a step-up transformer increases voltage through more turns on the secondary coil, while a step-down transformer decreases voltage.
Electronics

1) Electrostatic charges involve the attraction and repulsion of objects based on whether they have equal or opposite charges. Charges are used in photocopiers, dust precipitators, and defibrillators.
2) Electricity involves the flow of electrons through conductors. The resistance of materials can change based on factors like temperature. Common circuit components include resistors, diodes, and capacitors.
3) Digital and analog signals are used to transmit information. Digital signals use 1s and 0s while analog signals have continuously varying values. Multiplexing and sampling allow more data to be transmitted.
Jose and me ri rocks!!!

The document defines key terms related to food chains and food webs, including biomass, producer, consumer, herbivore, carnivore, predator, prey, decomposer. It provides an example food chain starting with plants as producers, then herbivores, primary and secondary consumers, and ending with a carnivore or tertiary consumer at the top. A diagram shows how energy transfers through a pyramid-shaped food web from producers to different consumer levels.
Food chain.ppt amit and mary

A food chain shows that plants are eaten by herbivores like rabbits, which are then eaten by carnivores like lions. Producers like plants make their own food and are the first link in the food chain, while consumers eat other organisms. Herbivores eat plants, carnivores eat meat, predators eat other animals, and prey are eaten by predators. Decomposers break down dead organisms. Humans, eagles and lions are at the top of food pyramids as they are rarely preyed upon by other animals.
Jose and me ri rocks!!!

The document defines key terms related to food chains and food webs, including biomass, producer, consumer, herbivore, carnivore, predator, prey, decomposer. It provides an example food chain starting with plants as producers, then herbivores, primary and secondary consumers, and ending with a carnivore or tertiary consumer at the top. A diagram shows how energy transfers through a pyramid-shaped food web from producers to different consumer levels.
Hello im sirsak and im neeth ^ ^

The document discusses key concepts related to food chains and biomass. It defines biomass as the total mass of a living thing excluding water. Biomass is composed of glucose and other substances produced through photosynthesis. A food chain diagrams how organisms feed on each other in an ecosystem, with producers like plants generating their own food, and consumers like herbivores and carnivores eating other organisms or animals. Decomposers feed on dead plants and animals. An example food chain is given of grass being eaten by a grasshopper and further up the chain.
Food chains

A food chain shows how each living thing gets its food, with arrows indicating what eats what. Carnivores are meat-eating animals like cats, lions, and snakes. Producers like plants make their own food through photosynthesis. Consumers eat other organisms, and can be herbivores that eat plants or predators that hunt other animals. Prey are the animals or plants that are hunted by predators.
Food chain.ppt amit and mary

A food chain shows that plants are eaten by herbivores like rabbits, which are then eaten by carnivores like lions. Producers like plants make their own food and are the first link in the food chain, while consumers eat other organisms. Herbivores eat plants, carnivores eat meat, predators eat other animals, and prey are eaten by predators. Decomposers break down dead organisms. Humans, eagles and lions are at the top of food pyramids as they are rarely preyed upon by other animals.
Food chain with alice

The document describes various food chains that exist on Earth and a hypothetical food chain on Mars. On Earth, producers like plants undergo photosynthesis at the bottom of the food chain, while consumers like herbivores and carnivores eat other organisms or animals higher up the chain. A food chain in the ocean includes phytoplankton, zooplankton, fish, and blue whales. The hypothetical Martian food chain positions Mares bacteria at the bottom, which are eaten by Mares aliens that feed on rocks. These aliens are preyed upon by the apex predator Sky beasts.
Sunni and nish foód¦¬presentaion

Biomass refers to the total mass of living organisms in an ecosystem, excluding water. Producers in a food chain are always plants, which produce their own food through photosynthesis. A food chain represents the predator-prey relationships between species in an ecosystem, with producers like plants being eaten by consumers such as snails, fish and ducks, and with carnivores preying on other animals and serving as predators for their prey.
Unit 2 8 Alcohols And Halogenoalkanes Notes

This document provides an overview of organic chemistry concepts related to alcohols and halogenoalkanes. It discusses:
1. The IUPAC system for naming organic compounds and provides examples of naming alcohols and halogenoalkanes.
2. Details the properties and reactions of alcohols, including combustion, reactions with sodium, oxidation, substitution, and how primary, secondary, and tertiary alcohols differ in reactivity.
3. Explains properties and reactions of halogenoalkanes such as hydrolysis, elimination, substitution, and how a test can identify primary, secondary, and tertiary halogenoalkanes.
4.
Unit 2 9 Mechanisms Notes

This document provides an overview of organic reaction mechanisms. It begins by explaining that chemical reactions can be studied in detail by examining how electrons move during the reaction, known as the reaction mechanism. It then discusses different types of organic reactions like addition, elimination, and substitution. The document also categorizes reagents as nucleophiles or electrophiles. It explains how single bonds can break through homolytic or heterolytic fission. Finally, it discusses free radical chemistry and how CFCs catalyze the destruction of stratospheric ozone through a free radical chain reaction.
Solar Elyse And Jeong

The document discusses solar energy, including what it is, how it is harnessed, where it comes from, its advantages and disadvantages, and some of its uses. Solar energy comes from the sun and can be used to generate electricity or heat water. It is harnessed using solar panels that convert sunlight into electrical energy or use it to heat fluids. While a renewable source of energy, solar power has initial costs and requires large land areas for panels, and production of panels can release toxic byproducts if not properly handled.
Future Transport Nishan And Sunny

Alternative fuels are fuels other than conventional fossil fuels like oil, coal, and natural gas. Some common alternative fuels include biodiesel, ethanol, hydrogen, and electricity from batteries, fuel cells, solar, or wind power. Alternative fuels are needed because fossil fuel resources are running out and renewable alternatives are needed. Battery electric vehicles use electric motors powered by rechargeable battery packs instead of gasoline engines. They have less pollution than gas cars but slower charging times and speeds. Solar cars are powered by solar panels that convert sunlight directly into electrical energy for electric motors. They produce zero emissions but are expensive and can only be used during the day. Fuel cells produce electricity through chemical reactions without combustion, which can be used to power electric
Nuclear And Geothermal Neeth And Sirsak

Nuclear energy generates electricity by splitting or fusing atomic nuclei, releasing energy according to Einstein's mass-energy equivalence formula. Geothermal energy taps heat from the earth's core to generate electricity. Both use heat to generate electricity, with nuclear providing low-cost, low-pollution power. France generates over 75% of its electricity from nuclear energy due to energy security policies. Geothermal energy is also used for district heating and bathing.
Wind Alice, Amit And Amy

Wind energy is a renewable source of energy that is harnessed using wind turbines located in wind farms. Wind turbines convert the kinetic energy of wind into electrical energy, with the turbine blades spun by wind currents that are ultimately driven by the sun. Wind power is being used increasingly all over the world, with wind farms established in locations like the UK and Australia to generate electricity from wind energy.
Biomass Harishan And Benji

Biomass is biological material from living or recently living organisms that can be used as an energy source. Examples of biomass include wood, trees, shrubs, crops, grasses, algae, and animal and food waste. Biomass is a renewable source of energy because it comes from sunlight that plants absorb to grow, and biomass can be regrown as it is used. Biomass is burned in boilers to produce steam that turns turbines and generates electricity.
More from M F Ebden (20)
Recently uploaded
Pengantar Penggunaan Flutter - Dart programming language1.pptx

Pengantar Penggunaan Flutter - Dart programming language1.pptx
Wound healing PPT

This document provides an overview of wound healing, its functions, stages, mechanisms, factors affecting it, and complications.
A wound is a break in the integrity of the skin or tissues, which may be associated with disruption of the structure and function.
Healing is the body’s response to injury in an attempt to restore normal structure and functions.
Healing can occur in two ways: Regeneration and Repair
There are 4 phases of wound healing: hemostasis, inflammation, proliferation, and remodeling. This document also describes the mechanism of wound healing. Factors that affect healing include infection, uncontrolled diabetes, poor nutrition, age, anemia, the presence of foreign bodies, etc.
Complications of wound healing like infection, hyperpigmentation of scar, contractures, and keloid formation.
A Visual Guide to 1 Samuel | A Tale of Two Hearts

These slides walk through the story of 1 Samuel. Samuel is the last judge of Israel. The people reject God and want a king. Saul is anointed as the first king, but he is not a good king. David, the shepherd boy is anointed and Saul is envious of him. David shows honor while Saul continues to self destruct.
BBR 2024 Summer Sessions Interview Training

Qualitative research interview training by Professor Katrina Pritchard and Dr Helen Williams
The History of Stoke Newington Street Names

Presented at the Stoke Newington Literary Festival on 9th June 2024
www.StokeNewingtonHistory.com
How to Make a Field Mandatory in Odoo 17

In Odoo, making a field required can be done through both Python code and XML views. When you set the required attribute to True in Python code, it makes the field required across all views where it's used. Conversely, when you set the required attribute in XML views, it makes the field required only in the context of that particular view.
Walmart Business+ and Spark Good for Nonprofits.pdf

"Learn about all the ways Walmart supports nonprofit organizations.
You will hear from Liz Willett, the Head of Nonprofits, and hear about what Walmart is doing to help nonprofits, including Walmart Business and Spark Good. Walmart Business+ is a new offer for nonprofits that offers discounts and also streamlines nonprofits order and expense tracking, saving time and money.
The webinar may also give some examples on how nonprofits can best leverage Walmart Business+.
The event will cover the following::
Walmart Business + (https://business.walmart.com/plus) is a new shopping experience for nonprofits, schools, and local business customers that connects an exclusive online shopping experience to stores. Benefits include free delivery and shipping, a 'Spend Analytics” feature, special discounts, deals and tax-exempt shopping.
Special TechSoup offer for a free 180 days membership, and up to $150 in discounts on eligible orders.
Spark Good (walmart.com/sparkgood) is a charitable platform that enables nonprofits to receive donations directly from customers and associates.
Answers about how you can do more with Walmart!"
Benner "Expanding Pathways to Publishing Careers"

This presentation was provided by Rebecca Benner, Ph.D., of the American Society of Anesthesiologists, for the second session of NISO's 2024 Training Series "DEIA in the Scholarly Landscape." Session Two: 'Expanding Pathways to Publishing Careers,' was held June 13, 2024.
RHEOLOGY Physical pharmaceutics-II notes for B.pharm 4th sem students

Physical pharmaceutics notes for B.pharm students
Chapter wise All Notes of First year Basic Civil Engineering.pptx

Chapter wise All Notes of First year Basic Civil Engineering
Syllabus
Chapter-1
Introduction to objective, scope and outcome the subject
Chapter 2
Introduction: Scope and Specialization of Civil Engineering, Role of civil Engineer in Society, Impact of infrastructural development on economy of country.
Chapter 3
Surveying: Object Principles & Types of Surveying; Site Plans, Plans & Maps; Scales & Unit of different Measurements.
Linear Measurements: Instruments used. Linear Measurement by Tape, Ranging out Survey Lines and overcoming Obstructions; Measurements on sloping ground; Tape corrections, conventional symbols. Angular Measurements: Instruments used; Introduction to Compass Surveying, Bearings and Longitude & Latitude of a Line, Introduction to total station.
Levelling: Instrument used Object of levelling, Methods of levelling in brief, and Contour maps.
Chapter 4
Buildings: Selection of site for Buildings, Layout of Building Plan, Types of buildings, Plinth area, carpet area, floor space index, Introduction to building byelaws, concept of sun light & ventilation. Components of Buildings & their functions, Basic concept of R.C.C., Introduction to types of foundation
Chapter 5
Transportation: Introduction to Transportation Engineering; Traffic and Road Safety: Types and Characteristics of Various Modes of Transportation; Various Road Traffic Signs, Causes of Accidents and Road Safety Measures.
Chapter 6
Environmental Engineering: Environmental Pollution, Environmental Acts and Regulations, Functional Concepts of Ecology, Basics of Species, Biodiversity, Ecosystem, Hydrological Cycle; Chemical Cycles: Carbon, Nitrogen & Phosphorus; Energy Flow in Ecosystems.
Water Pollution: Water Quality standards, Introduction to Treatment & Disposal of Waste Water. Reuse and Saving of Water, Rain Water Harvesting. Solid Waste Management: Classification of Solid Waste, Collection, Transportation and Disposal of Solid. Recycling of Solid Waste: Energy Recovery, Sanitary Landfill, On-Site Sanitation. Air & Noise Pollution: Primary and Secondary air pollutants, Harmful effects of Air Pollution, Control of Air Pollution. . Noise Pollution Harmful Effects of noise pollution, control of noise pollution, Global warming & Climate Change, Ozone depletion, Greenhouse effect
Text Books:
1. Palancharmy, Basic Civil Engineering, McGraw Hill publishers.
2. Satheesh Gopi, Basic Civil Engineering, Pearson Publishers.
3. Ketki Rangwala Dalal, Essentials of Civil Engineering, Charotar Publishing House.
4. BCP, Surveying volume 1
BÀI TẬP BỔ TRỢ TIẾNG ANH 8 CẢ NĂM - GLOBAL SUCCESS - NĂM HỌC 2023-2024 (CÓ FI...

BÀI TẬP BỔ TRỢ TIẾNG ANH 8 CẢ NĂM - GLOBAL SUCCESS - NĂM HỌC 2023-2024 (CÓ FI...Nguyen Thanh Tu Collection
https://app.box.com/s/y977uz6bpd3af4qsebv7r9b7s21935vdWhat is Digital Literacy? A guest blog from Andy McLaughlin, University of Ab...

What is Digital Literacy? A guest blog from Andy McLaughlin, University of Aberdeen
Stack Memory Organization of 8086 Microprocessor

The stack memory organization of 8086 microprocessor.
Gender and Mental Health - Counselling and Family Therapy Applications and In...

A proprietary approach developed by bringing together the best of learning theories from Psychology, design principles from the world of visualization, and pedagogical methods from over a decade of training experience, that enables you to: Learn better, faster!
BÀI TẬP DẠY THÊM TIẾNG ANH LỚP 7 CẢ NĂM FRIENDS PLUS SÁCH CHÂN TRỜI SÁNG TẠO ...

BÀI TẬP DẠY THÊM TIẾNG ANH LỚP 7 CẢ NĂM FRIENDS PLUS SÁCH CHÂN TRỜI SÁNG TẠO ...Nguyen Thanh Tu Collection
https://app.box.com/s/qhtvq32h4ybf9t49ku85x0n3xl4jhr15Recently uploaded (20)
Pengantar Penggunaan Flutter - Dart programming language1.pptx

Pengantar Penggunaan Flutter - Dart programming language1.pptx
Walmart Business+ and Spark Good for Nonprofits.pdf

Walmart Business+ and Spark Good for Nonprofits.pdf
RHEOLOGY Physical pharmaceutics-II notes for B.pharm 4th sem students

RHEOLOGY Physical pharmaceutics-II notes for B.pharm 4th sem students
Chapter wise All Notes of First year Basic Civil Engineering.pptx

Chapter wise All Notes of First year Basic Civil Engineering.pptx
BÀI TẬP BỔ TRỢ TIẾNG ANH 8 CẢ NĂM - GLOBAL SUCCESS - NĂM HỌC 2023-2024 (CÓ FI...

BÀI TẬP BỔ TRỢ TIẾNG ANH 8 CẢ NĂM - GLOBAL SUCCESS - NĂM HỌC 2023-2024 (CÓ FI...
What is Digital Literacy? A guest blog from Andy McLaughlin, University of Ab...

What is Digital Literacy? A guest blog from Andy McLaughlin, University of Ab...
NEWSPAPERS - QUESTION 1 - REVISION POWERPOINT.pptx

NEWSPAPERS - QUESTION 1 - REVISION POWERPOINT.pptx
Gender and Mental Health - Counselling and Family Therapy Applications and In...

Gender and Mental Health - Counselling and Family Therapy Applications and In...
BÀI TẬP DẠY THÊM TIẾNG ANH LỚP 7 CẢ NĂM FRIENDS PLUS SÁCH CHÂN TRỜI SÁNG TẠO ...

BÀI TẬP DẠY THÊM TIẾNG ANH LỚP 7 CẢ NĂM FRIENDS PLUS SÁCH CHÂN TRỜI SÁNG TẠO ...
C5e Gas Volumes
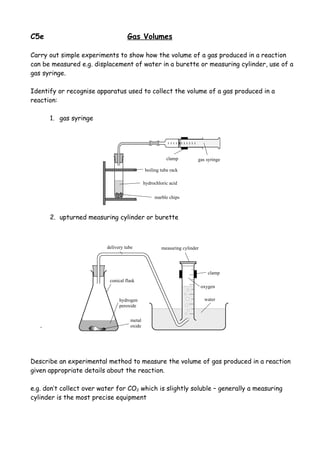
- 1. C5e Gas Volumes Carry out simple experiments to show how the volume of a gas produced in a reaction can be measured e.g. displacement of water in a burette or measuring cylinder, use of a gas syringe. Identify or recognise apparatus used to collect the volume of a gas produced in a reaction: 1. gas syringe clamp gas syringe boiling tube rack hydrochloric acid marble chips 2. upturned measuring cylinder or burette delivery tube measuring cylinder clamp conical flask oxygen hydrogen water peroxide metal . oxide Describe an experimental method to measure the volume of gas produced in a reaction given appropriate details about the reaction. e.g. don’t collect over water for CO2 which is slightly soluble – generally a measuring cylinder is the most precise equipment
- 2. Reactions Involving Gases Examples Mg + 2HCl MgCl2 + H2 CaCO3 + 2HCl CaCl2 + H2O + CO2 • A reaction stops when one of the reactants is all used up. • The more reactant you use the higher the volume of gas made • The limiting reactant is the one that is used up first • The other reactant is said to be in excess i.e. more than enough • A reaction stops when the limiting reactant has been used up • The volume of gas produced is directly proportional to the amount of the limiting reactant present Questions 1 gram of magnesium is reacted in excess hydrochloric acid. It took 30s for the reaction to stop and 52 cm3 of gas was collected. 1. What would you observe that tells you a reaction is taking place? 2. How would you know when the reaction had finished? Give 2 observations. 3. Why does the reaction stop? 4. Which is the limiting reactant? 5. How much gas would be collected after 60s? 6. If 2 grams of magnesium were used (and the acid was still in excess) how much gas would be collected by the time the reaction stopped? 20cm3 of hydrochloric acid was reacted with excess marble chips. 75cm3 of gas had been collected when the reaction stopped 7. Which is the limiting reactant? 8. How much gas would be collected if marble powder was used instead of marble chips? 9. How much gas would be collected 10cm3 of acid were used?
- 3. Reactions Involving Gases Examples Mg + 2HCl MgCl2 + H2 CaCO3 + 2HCl CaCl2 + H2O + CO2 • A reaction stops when _________________________________________ • The more reactant you use the __________________ the volume of gas made • The _____________________ reactant is the one that is used up first • The other reactant is said to be in ________________ i.e. more than enough • A reaction stops when the ____________________ reactant has been used up • The volume of gas produced is __________________ proportional to the amount of the limiting reactant present Questions 1 gram of magnesium is reacted in excess hydrochloric acid. It took 30s for the reaction to stop and 52 cm3 of gas was collected. 1. What would you observe that tells you a reaction is taking place? 2. How would you know when the reaction had finished? Give 2 observations. 3. Why does the reaction stop? 4. Which is the limiting reactant? 5. How much gas would be collected after 60s? 6. If 2 grams of magnesium were used (and the acid was still in excess) how much gas would be collected by the time the reaction stopped? 20cm3 of hydrochloric acid was reacted with excess marble chips. 75cm3 of gas had been collected when the reaction stopped 7. Which is the limiting reactant? 8. How much gas would be collected if marble powder was used instead of marble chips? 9. How much gas would be collected if 10cm3 of acid were used?
- 4. Investigating Rates of Reaction CaCO3 + 2HCl CaCl2 + H2O + CO2 Here are the results for the above reaction where HCl was reacted with excess marble chips, CaCO3, using different concentrations of acid. Concentration (mol/dm3) Time 0.8 0.6 0.4 0.2 (seconds) 0 0 0 0 0 15 18 8 4 4 30 30 15 9 7 45 42 21 11 12 60 49 22 13 15 75 50 30 15 20 90 59 34 20 21 105 62 37 23 23 120 66 39 27 24 135 67 40 30 24 150 68 41 31 24 165 69 42 33 24 180 70 43 34 24 1. Plot a graph using the results put time on the x axis 2. Describe the shape of the curves. 3. For the 0.8mol/dm3 solution label the fastest point on the graph? 4. How do we know the rate decreases (slows down) near the end? 5. How do we know when the reaction as stopped? 6. The gradient of this graph gives the rate of reaction at any point. Draw a tangent to the initial slope of your curve 7. Work out the gradient (y/x) of this tangent – this is your initial rate Now, given the results shown in the table below, determine the initial rates of reaction for the 4 different concentrations:
- 5. HIGHER Given the knowledge that one mole of gas molecules occupy a volume of 24 dm3 at room temperature and pressure, use it to calculate the volumes of samples of gases. Perform calculations involving gas volumes and number of moles.
- 6. Drawing Graphs and Making Predictions 1. Hydrochloric acid reacting with magnesium ribbon: a) Sketch a curve to show what happens to the volume of gas collected over a period of time when there is an increase in temperature. low temperature b) What factors must be kept constant? c) Explain the shape of the curve that you have drawn in as much detail as you can, refer to the gradient and final volume. d) What has happened to the rate? Explain why in terms of the collision theory in as much detail as you can. 2. Marble chips reacting in acid: a) Sketch a curve to show what happens to small marble the volume of gas collected over a period chips of time when larger marble chips are used. b) What factors must be kept constant? c) What has happened to the rate? Explain why in terms of the collision theory.
- 7. 3. Hydrochloric acid reacting with magnesium ribbon: a) Sketch 2 curves. One to show what happens to the volume of gas collected over a period of time when a more dilute solution is used and one to show a more concentrated solution. c) What has happened to the rate? Explain why in terms of the collision theory. 4. a) The graph below shows the volume of gas collected when 20cm3 of HCl were reacted with a large excess of marble chips. Sketch the shape of the graph that you would get if 40cm3 of HCl was reacted with a large excess. 40 Volume of CO2 30 produced (cm3) 20 10 Time (s) b) Explain your answer
- 8. Carry out experiments to measure the mass of a gas being produced during a reaction e.g. marble and acid and/or thermal decomposition of zinc carbonate. Describe that measurement of change of mass may be used to monitor the amount of gas made in a reaction. Describe an experimental method to measure the mass of gas produced in a reaction given appropriate details about the reaction. NO HIGHER