1. The document describes an experiment to determine the equilibrium constant Kc for the esterification reaction of ethanoic acid and ethanol.
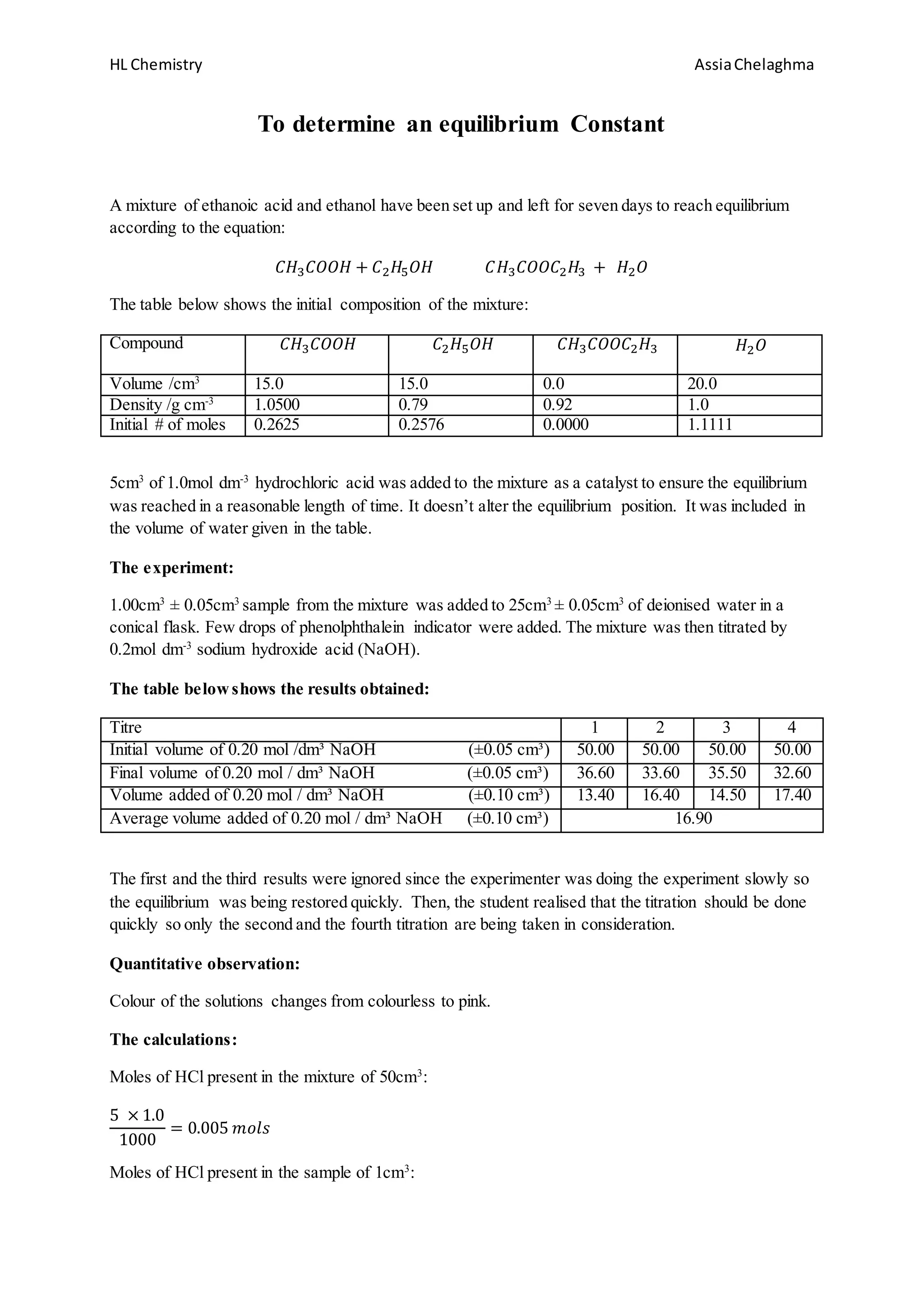
2. Initial concentrations of reactants and products were measured and the mixture was allowed to reach equilibrium. Samples were then titrated with sodium hydroxide and the volume added was used to calculate Kc.
3. The experimental value of Kc was found to be 4.80, which has a 20% error from the theoretical value of 4.07 given by the teacher. Sources of error are discussed.