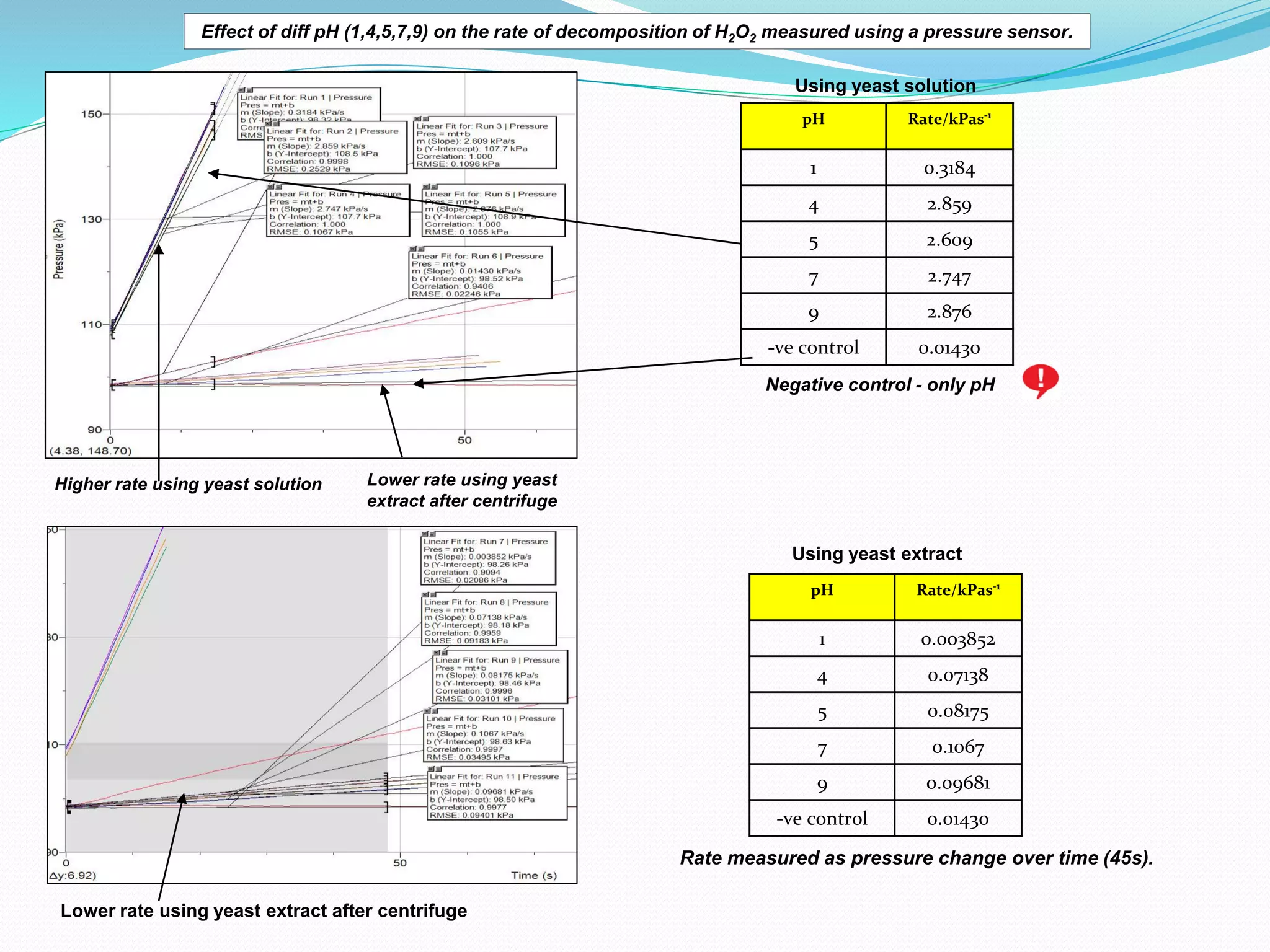
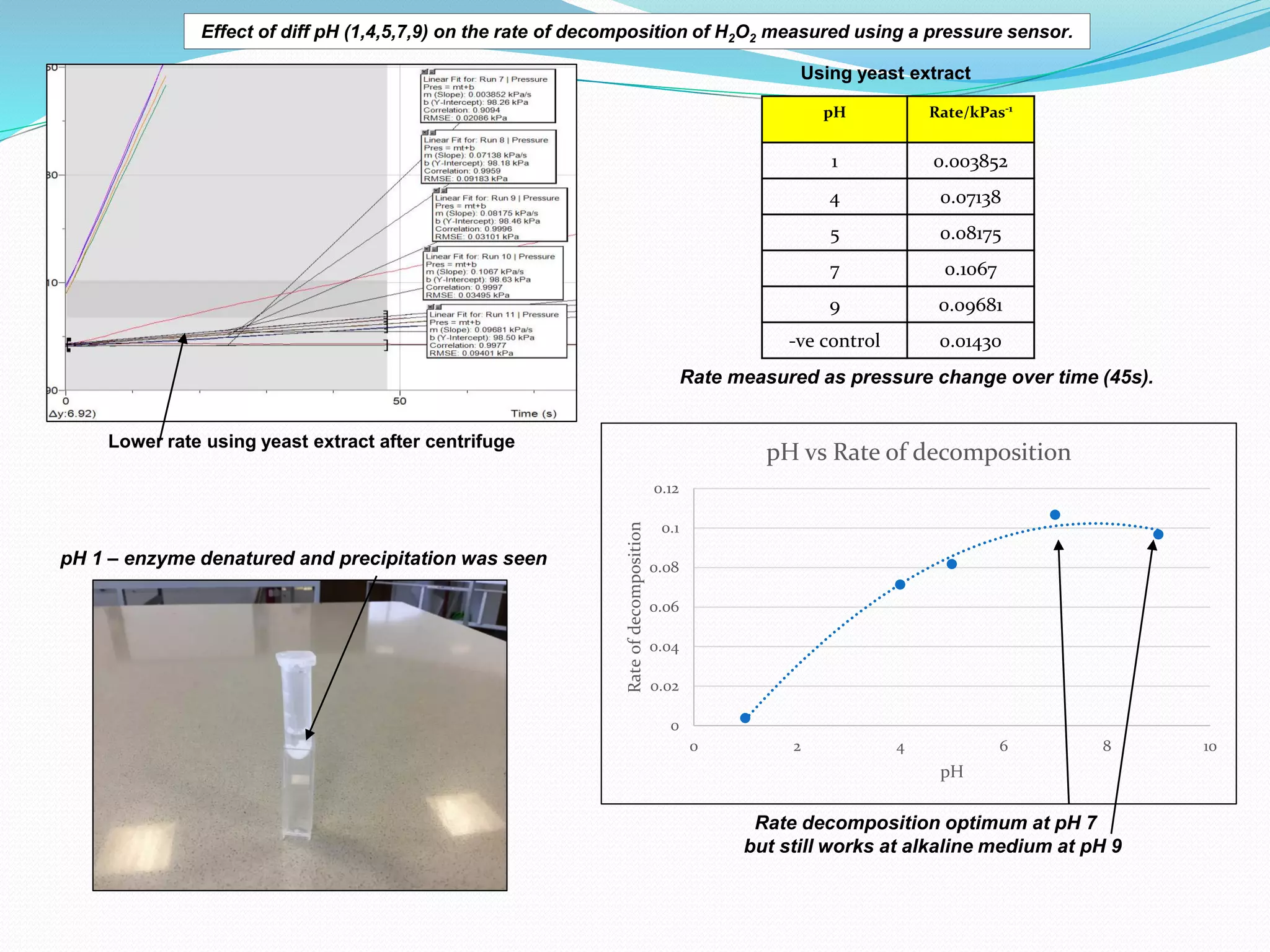
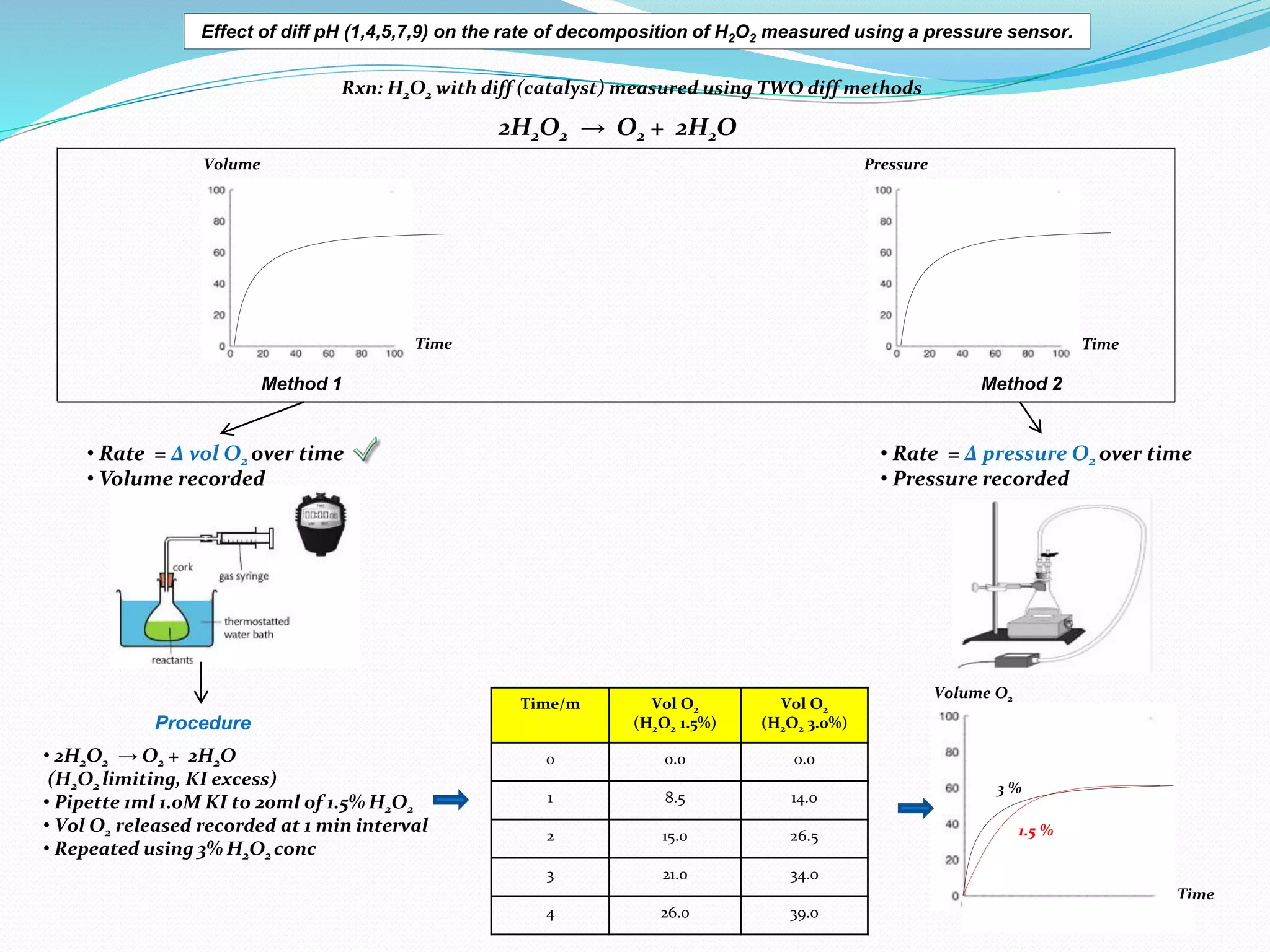
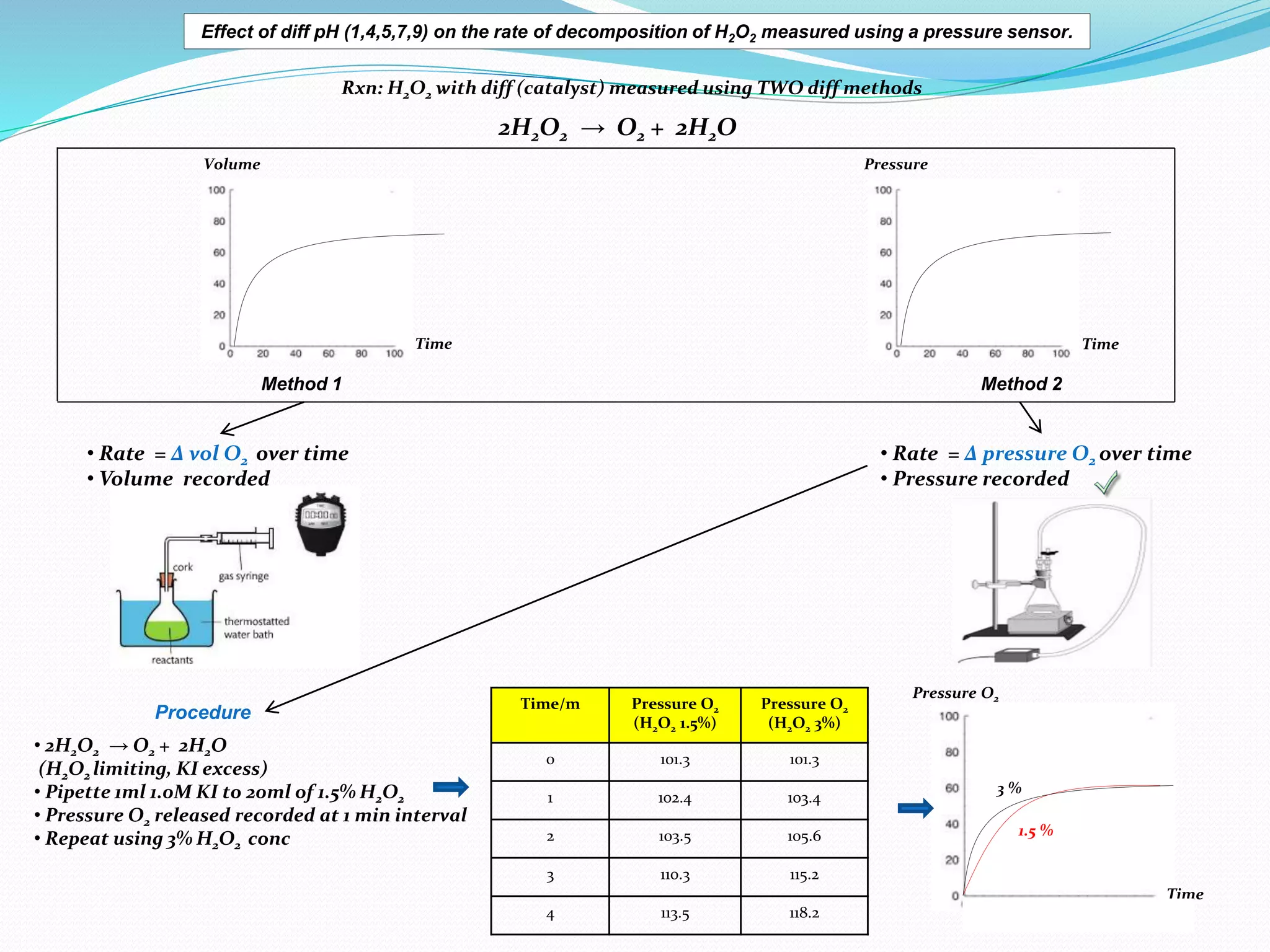
The document details an experiment investigating the effect of pH on the rate of hydrogen peroxide decomposition catalyzed by yeast-extracted catalase. Various pH levels (1, 4, 5, 7, 9) were tested, with results showing optimal activity at pH 7, while denaturation occurred at pH 1. The methodology included measuring oxygen production using a pressure sensor, with results indicating variable decomposition rates across different pH values.