This document summarizes catalytic hydrogenation, including:
- Heterogenous catalytic hydrogenation occurs on metal surfaces like Ni, Pd, Pt.
- Homogenous hydrogenation uses complexes like Wilkinson's catalyst in solution.
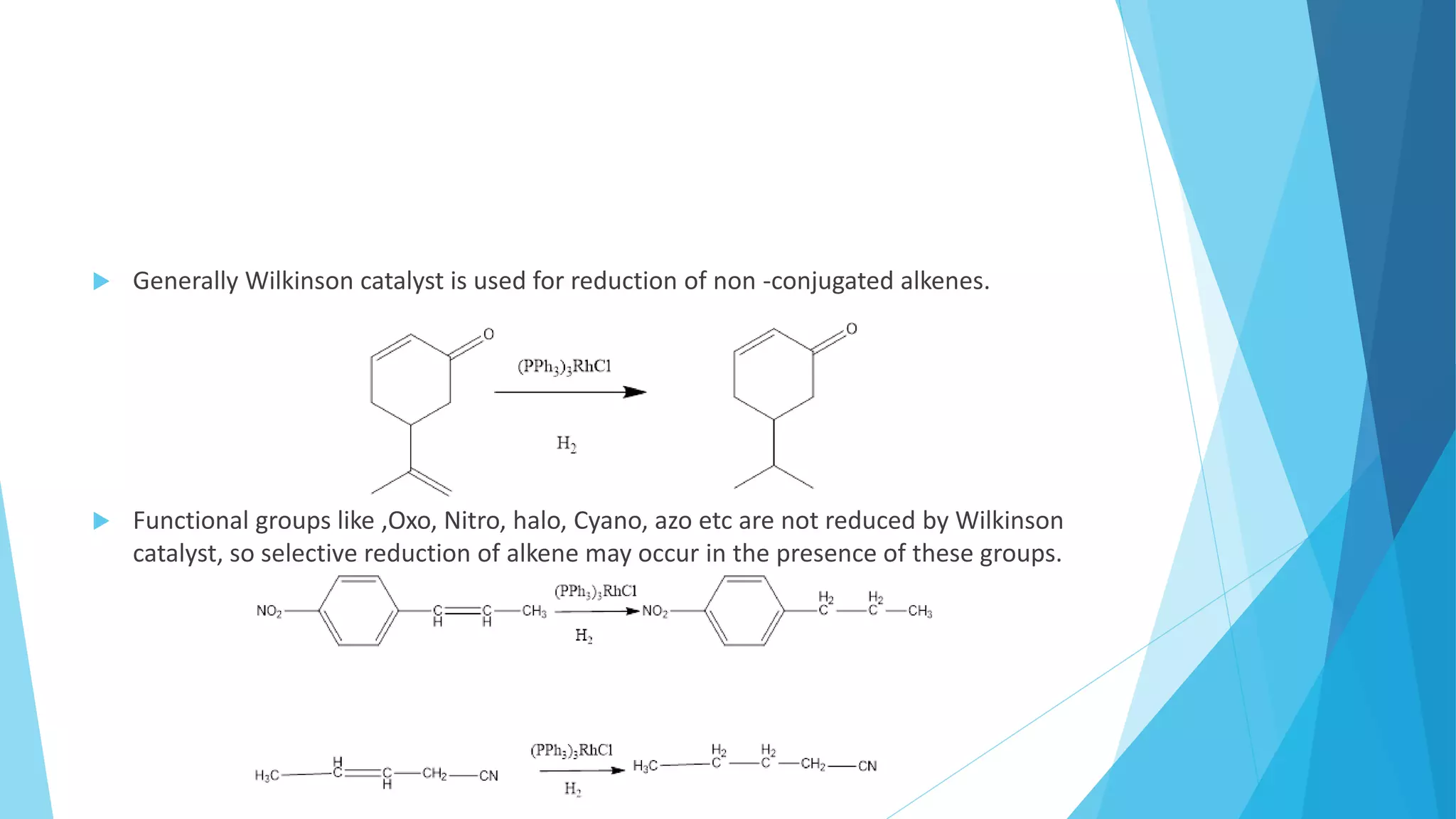
- Rate of reduction decreases with increased substitution. Selective reduction of double bonds is possible.
- Catalytic hydrogenation is stereospecific, giving syn-addition and racemic mixtures for cis-alkenes.










![Homogenous hydrogenation
It is proceed by Wilkinson catalyst and Hydrogen. The use of complexes of Rhodium or
Ruthenium as catalysts enables hydrogenation to be carried out in homogenous
solution.
[(PPh3)3RhCl] = Wilkinson catalyst is a well known organometallic compound.
Chloridotris(triphenyl phosphine) Rhodium[I]](https://image.slidesharecdn.com/thippeswamyba-220718001420-0ef54c0b/75/Thippeswamy-B-A-pptx-11-2048.jpg)