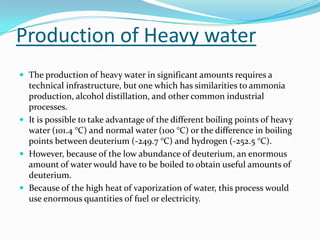
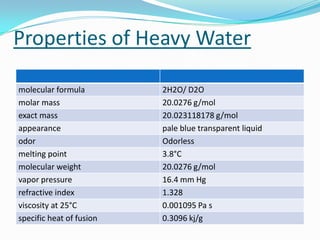
This document provides information about heavy water, including its history, properties, production, and applications. Heavy water consists of deuterium and oxygen and was first isolated in 1931. It is produced through distillation processes that take advantage of its slightly different boiling point from regular water. Heavy water is used as a moderator in nuclear reactors, slowing neutrons so they can induce fission in uranium-238 as well as uranium-235. It also has applications as a tracer in biological and chemical reactions.




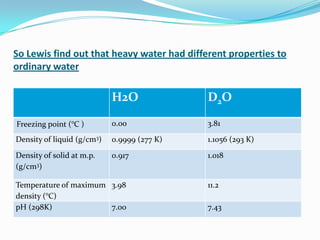

![Introduction
Heavy Water (D2O) (compound of Deuterium (D) and oxygen)
This is also known as Deuterium Oxide.
Deuterium (atomic mass 2) for normal hydrogen (H)
[due to presence of an extra neutron in the nucleus]
Heavy Water resembles in its physical and chemical properties to
ordinary water. (light Water)
But its nuclear properties makes it an extremely efficient material for
use as moderator in a nuclear reactor.](https://image.slidesharecdn.com/heavywater-130121020716-phpapp01/85/Heavy-water-7-320.jpg)