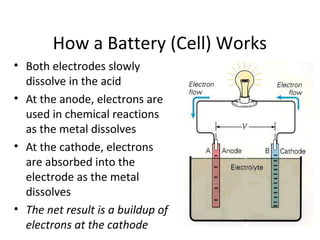
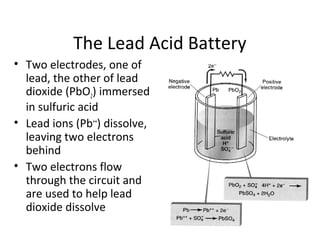
This document discusses different types of portable electric energy sources including batteries and fuel cells. It provides details on how batteries work including definitions of anode, cathode and amp-hours. Common battery types like alkaline, lead acid and lithium ion are described. The document also discusses electric vehicles and compares gasoline to battery technologies. Fuel cells are introduced as an alternative to batteries for electric vehicles that produce only water as a byproduct but have not been widely adopted due to cost and infrastructure challenges.