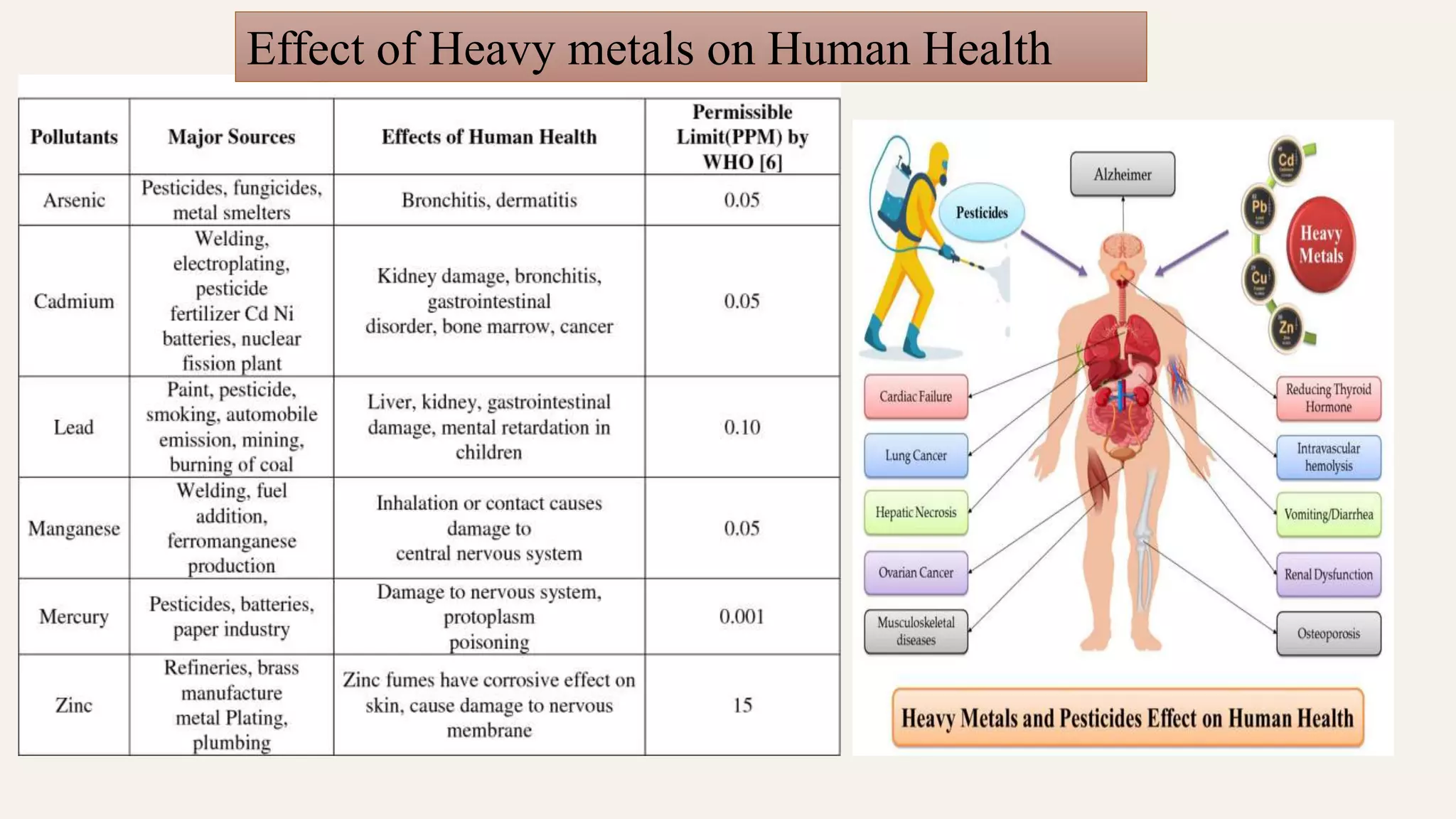
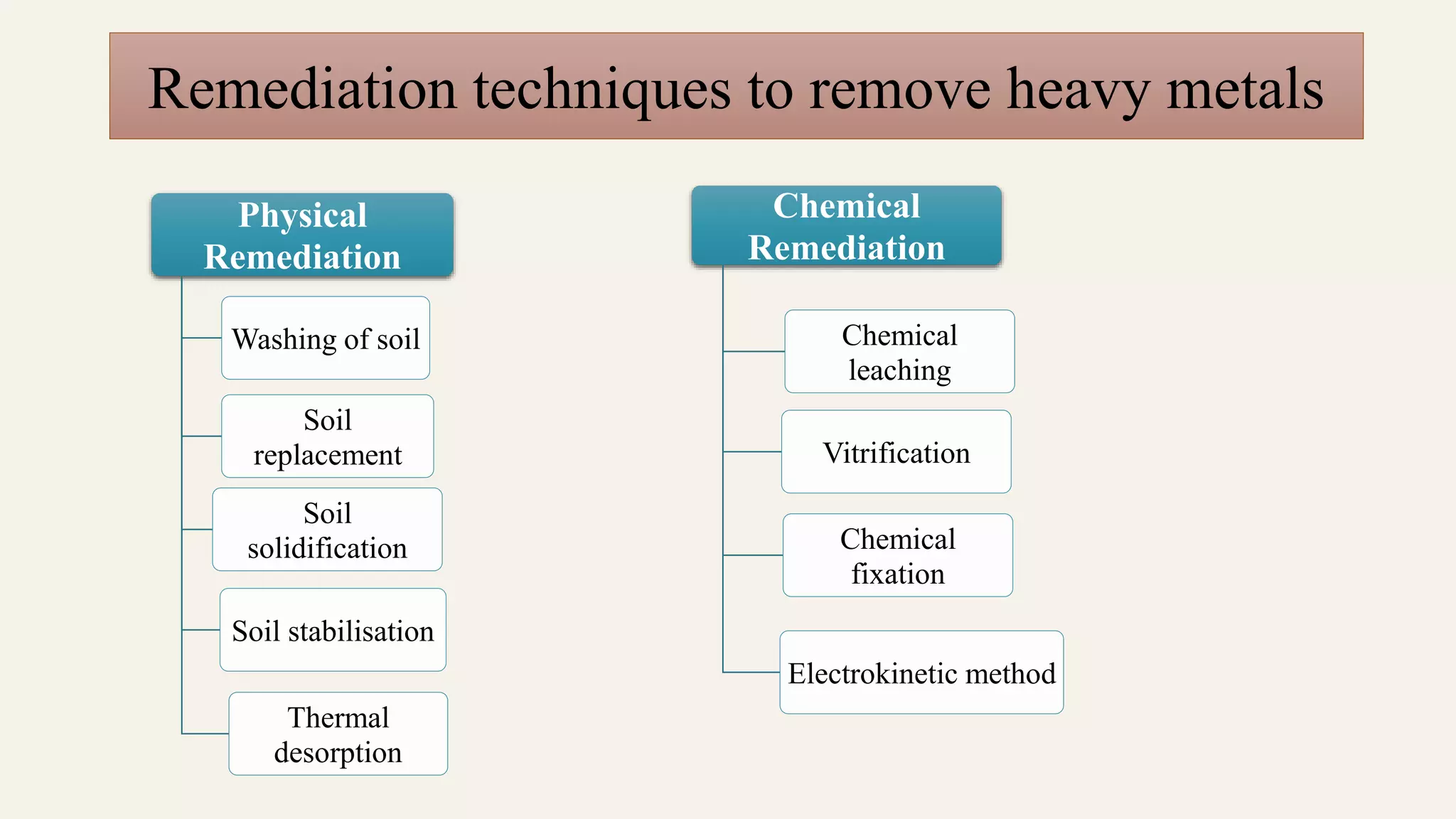
The document discusses heavy metal pollution, its sources, properties, and effects on the environment, particularly water, soil, and air quality, as well as its impact on plants and human health. It outlines the toxicological properties of heavy metals, the fate of metals in various environments, and highlights significant events of heavy metal poisoning. Additionally, it addresses remediation techniques, including biological options like phytoremediation and the role of microorganisms in transforming heavy metals.