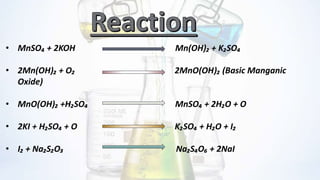
The document discusses the importance of dissolved oxygen (DO) in water for aquatic life and outlines methods for measuring it, particularly using Winkler's method. It explains that DO levels indicate water purity and can be affected by factors like temperature and salinity, with ideal quality being 4-6 mg/l. The document details the chemical reactions and procedures involved in the titration process for determining DO levels in water samples.