The document presents an extensive overview of halogens, detailing their general characteristics, preparation methods, chemical properties, and uses. It discusses their positions in the periodic table, various reactions with water, alkali, ammonia, and their oxidizing nature, along with comparative studies of haloacids. Additionally, it covers the testing methods for chlorine, bromine, and iodine, highlighting their applications in industries and as disinfectants.

![Syllabus according to NEB
9.5.1 General characteristics of halogens
9.5.2 Comparative study on preparation (no diagram and description is
required),
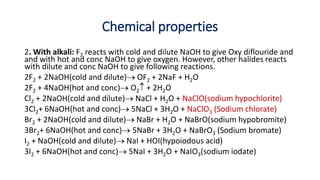
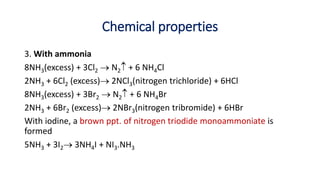
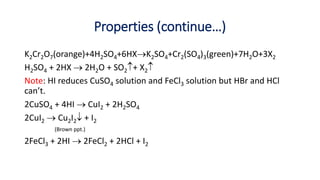
9.5.2.1 Chemical properties [with water, alkali, ammonia, oxidizing
character, bleaching action] and uses of halogens (Cl2, Br2 and I2)
9.5.3 Test for Cl2, Br2 and I2
9.5.4 Comparative study on preparation (no diagram and description is
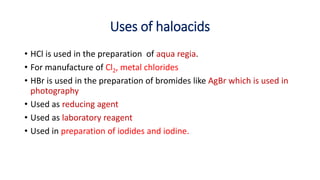
required), properties (reducing strength, acidic nature and solubility)
and uses of haloacids (HCl, HBr and HI)](https://image.slidesharecdn.com/halogens11-240510173458-ef7b5647/85/Halogens-class-11-chemistry-note-by-Bimochan-2-320.jpg)