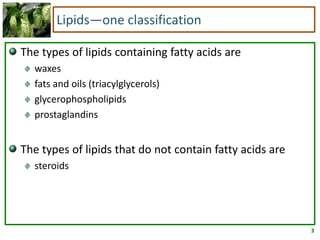
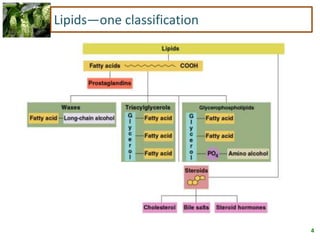
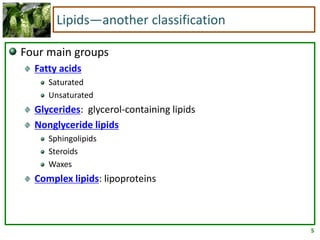
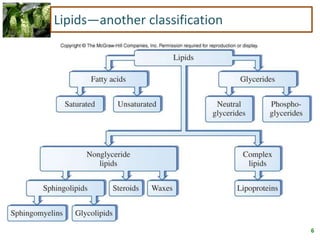
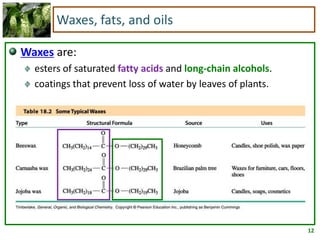
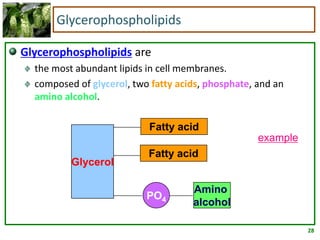
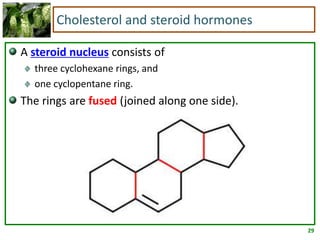
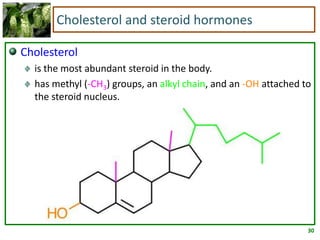
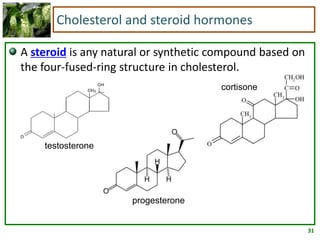
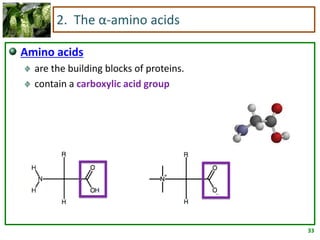
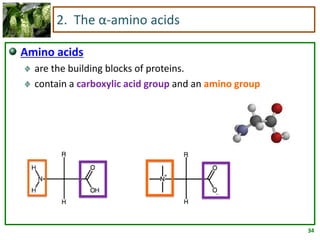
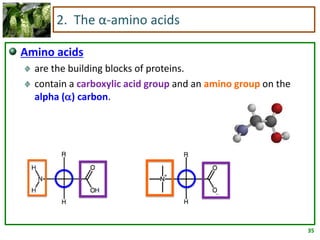
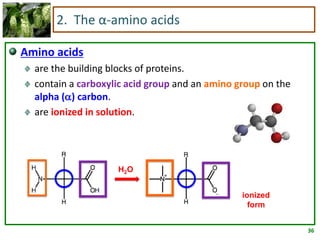
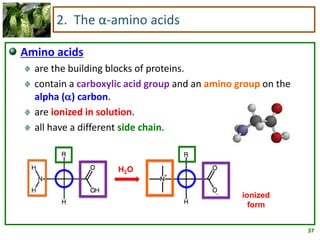
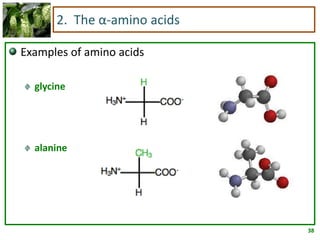
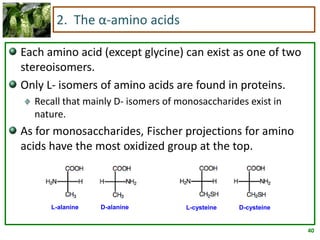
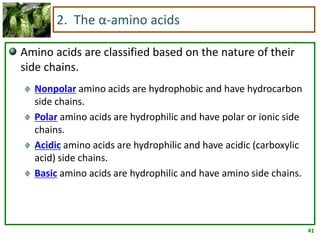
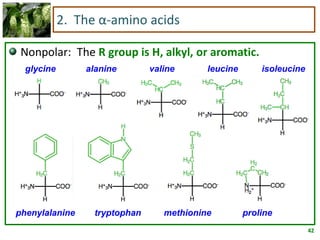
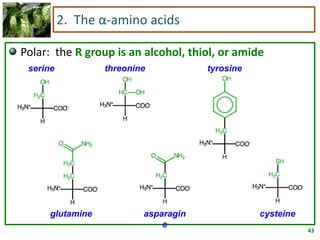
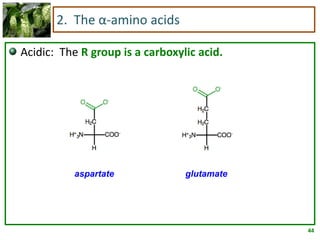
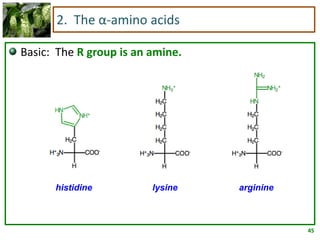
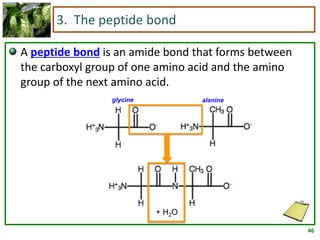
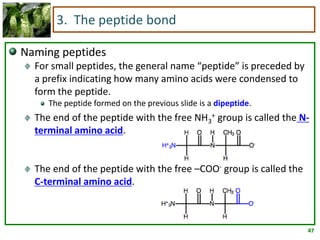
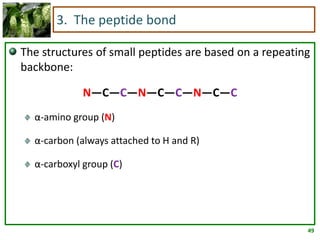
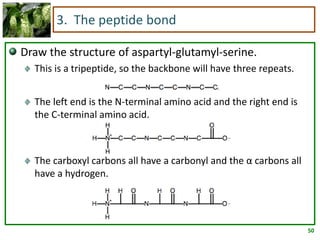
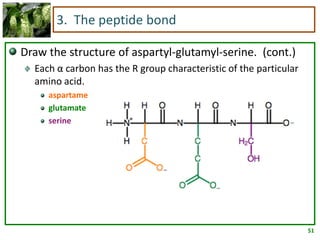
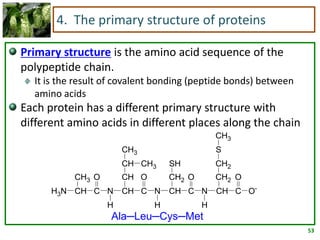
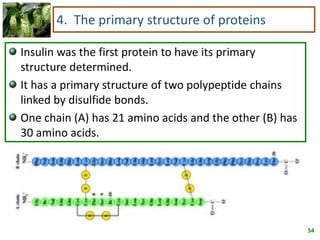
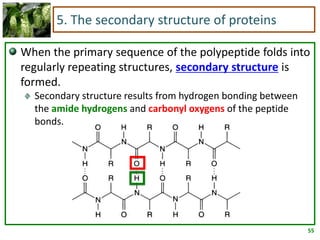
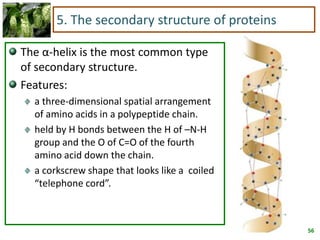
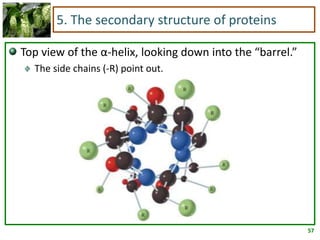
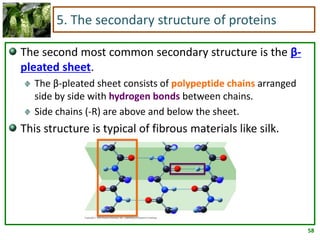
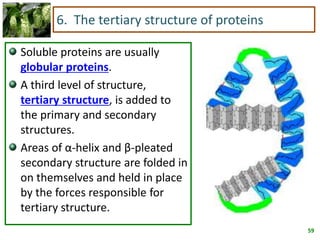
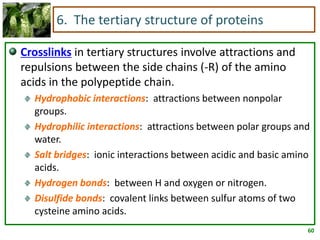
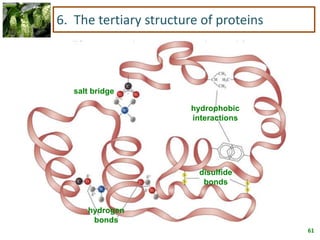
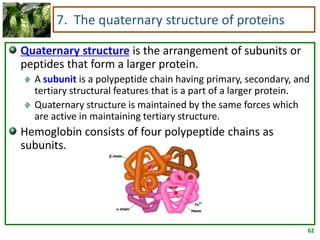
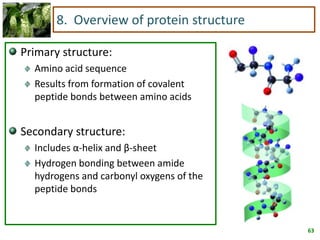
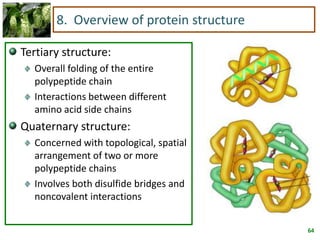
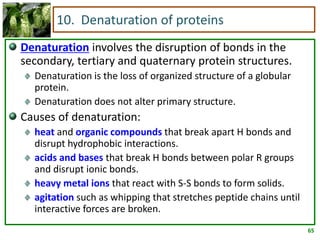
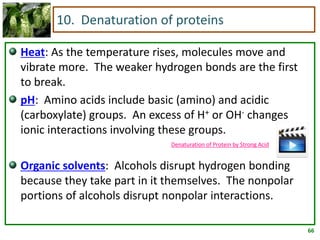
1) Lipids are biomolecules that include fatty acids and steroids. They are nonpolar and insoluble in water. The main types are fatty acids, glycerides, sphingolipids, and steroids.
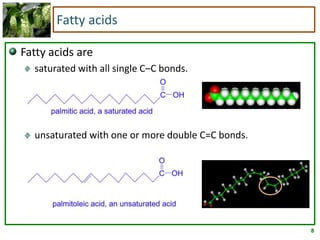
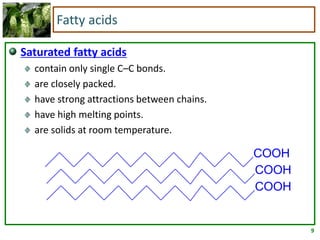
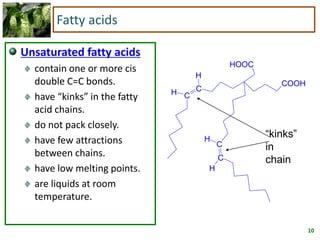
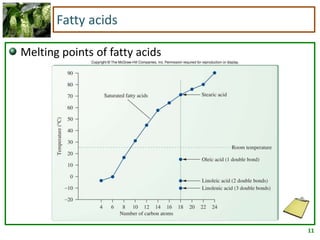
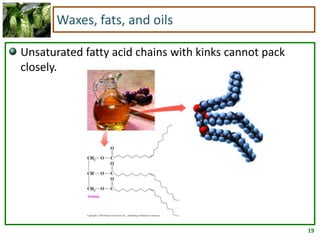
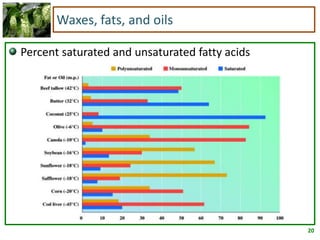
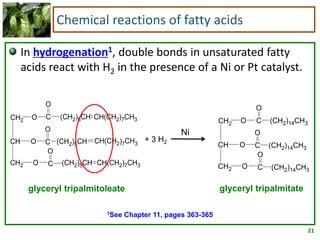
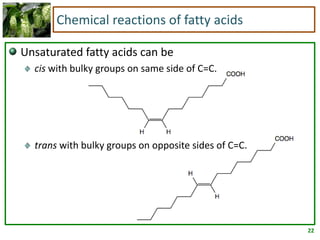
2) Fatty acids can be saturated or unsaturated. Saturated fatty acids are solid at room temperature due to close packing, while unsaturated fatty acids contain kinks that prevent close packing and result in lower melting points and liquid form.
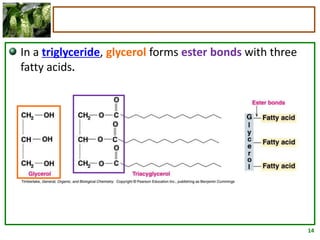
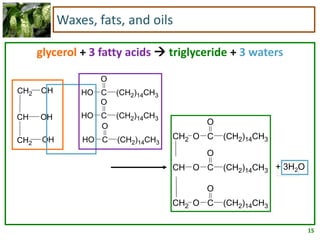
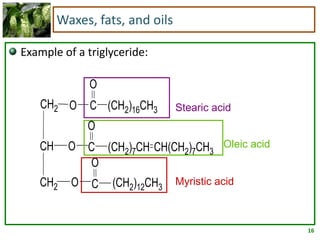
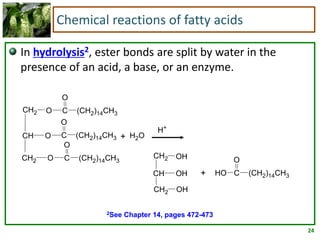
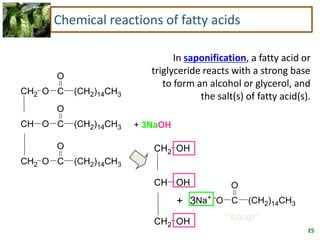
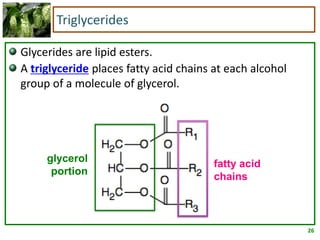
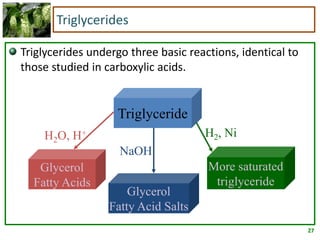
3) Triglycerides are the main form of lipids in fats and oils. They consist of a glycerol molecule bonded to three fatty acid molecules. Fats are solid at room temperature due to higher saturated fatty acid content,