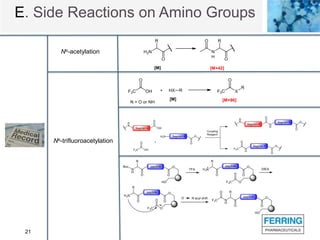
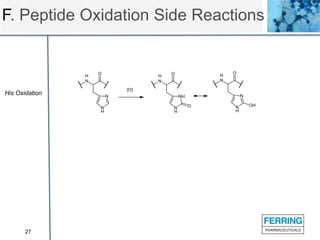
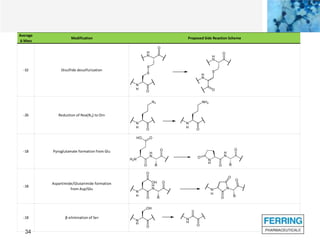
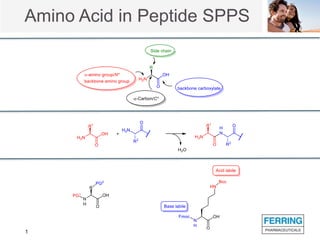
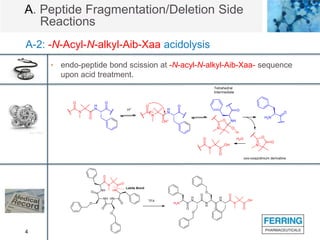
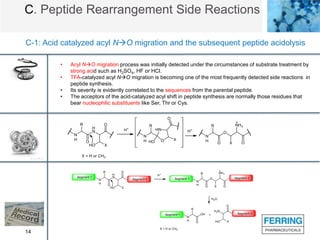
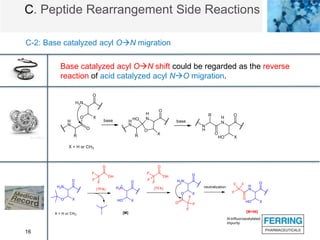
The document discusses various side reactions that can occur during solid phase peptide synthesis (SPPS), including peptide fragmentation, deletion reactions, β-elimination reactions, rearrangements, cyclizations, modifications of amino acid side chains, and oxidations. Specific examples are provided for each category, such as acidolysis of Asp-Pro bonds and N-acetyl-N-alkyl peptides, β-elimination of cysteine and phosphorylated residues, acid- or base-catalyzed acyl shifts, aspartimide and asparagine deamidation, and disulfide scrambling or degradation. Factors affecting the side reactions like acidity, sequence dependence, and excipient impurities are also examined.














![18
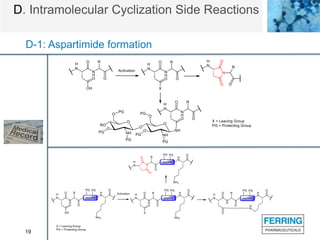
D. Intramolecular Cyclization Side Reactions
D-1: Aspartimide formation
• Asp converted to imide by repelling a H2O molecule [M-18].
• One of the most severe side reactions on peptides.
• Both acid and base-catalyzed.
• Occurred both in peptide synthesis, formulation, and storage.
• Sequence dependent -Asp-Xaa-
• Could also affect Glu, but to a much lesser extent (glutarimide).](https://image.slidesharecdn.com/8b0fcf88-29e4-4d9f-b9d3-a73dcf9a0e7c-160613144617/85/Peptide-Side-Reaction-19-320.jpg)
![20
D. Intramolecular Cyclization Side Reactions
D-2: Asn/Gln deamidation
• Asn/Gln-containing peptides are frequently involved in deamidation side reactions
• Amide side chains are converted into the corresponding carboxylates [M+1].
• Diverse mechanism.
• Both acid- and base-catalyzed.
• Sequence dependent -Asn-Xaa-](https://image.slidesharecdn.com/8b0fcf88-29e4-4d9f-b9d3-a73dcf9a0e7c-160613144617/85/Peptide-Side-Reaction-21-320.jpg)