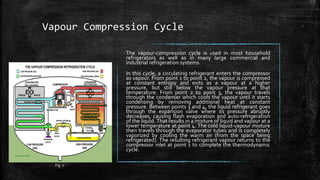
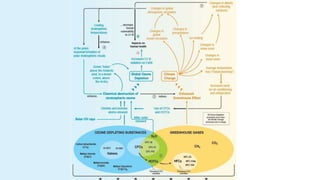
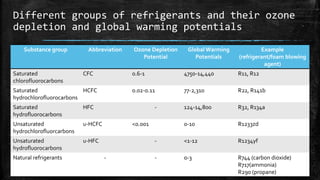
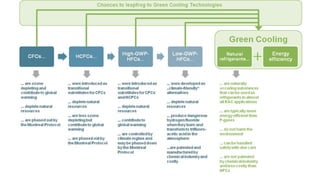
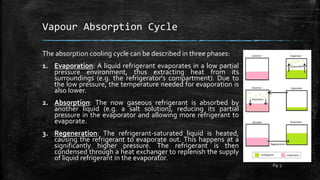
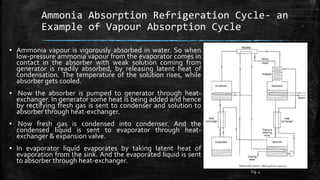
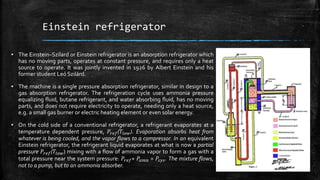
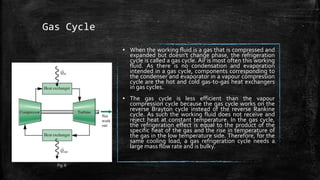
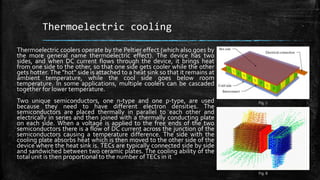
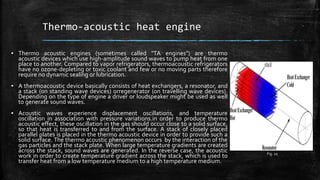
The document provides an overview of various refrigeration methods, including vapour compression and absorption cycles, highlighting their applications and environmental impacts, particularly regarding refrigerants. It discusses the evolution of refrigeration technology and its role in enhancing food storage and agricultural practices, as well as the shift towards natural and environmentally friendly refrigerants. The text also briefly describes alternate cooling technologies, such as thermoelectric, magnetic, and solar refrigeration systems.