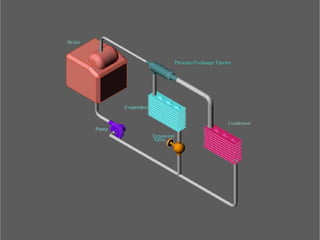
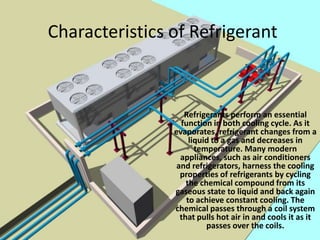
This document discusses refrigeration and air conditioning. It describes the refrigeration cycle process and different refrigeration methods including non-cyclic, cyclic, vapor compression, and gas cycle refrigeration. It defines a unit of refrigeration and discusses characteristics of refrigerants such as odor, color, boiling point, dangers, and benefits. The document was prepared by mechanical engineering students at Quaid-e-Awam University of Engineering, Science & Technology in Pakistan.