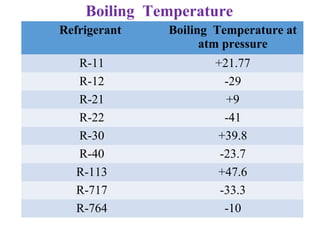
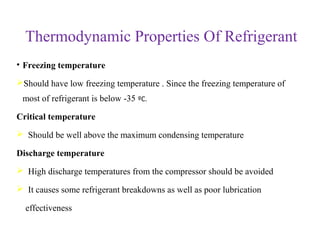
The document provides a detailed overview of refrigerants, defining them as fluids used for heat transfer in refrigeration systems. It classifies refrigerants into primary and secondary categories, discussing their types, properties, applications, and environmental impacts, particularly focusing on ozone depletion and global warming potential. Additionally, it outlines the chemical and thermodynamic characteristics of various refrigerants, highlighting their significance in refrigeration technology.