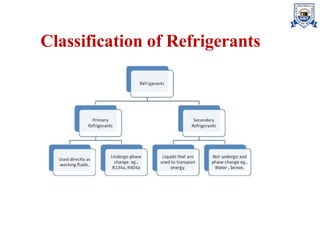
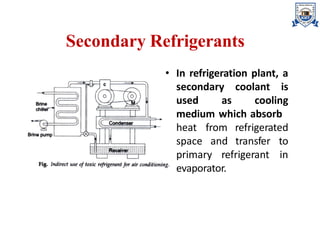
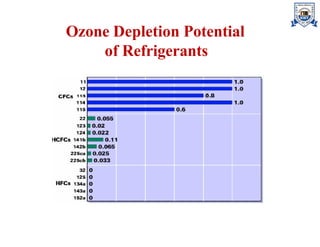
The document discusses various types of refrigerants used in refrigeration systems, categorizing them into primary and secondary refrigerants, and detailing their classifications, properties, and applications. It describes halocarbon, inorganic, azeotropic, zeotropic, hydrocarbon refrigerants, and the importance of refrigerant oils for compressor operation. Additionally, it addresses environmental considerations, safety classifications, and the impact of refrigerants on ozone depletion and global warming.