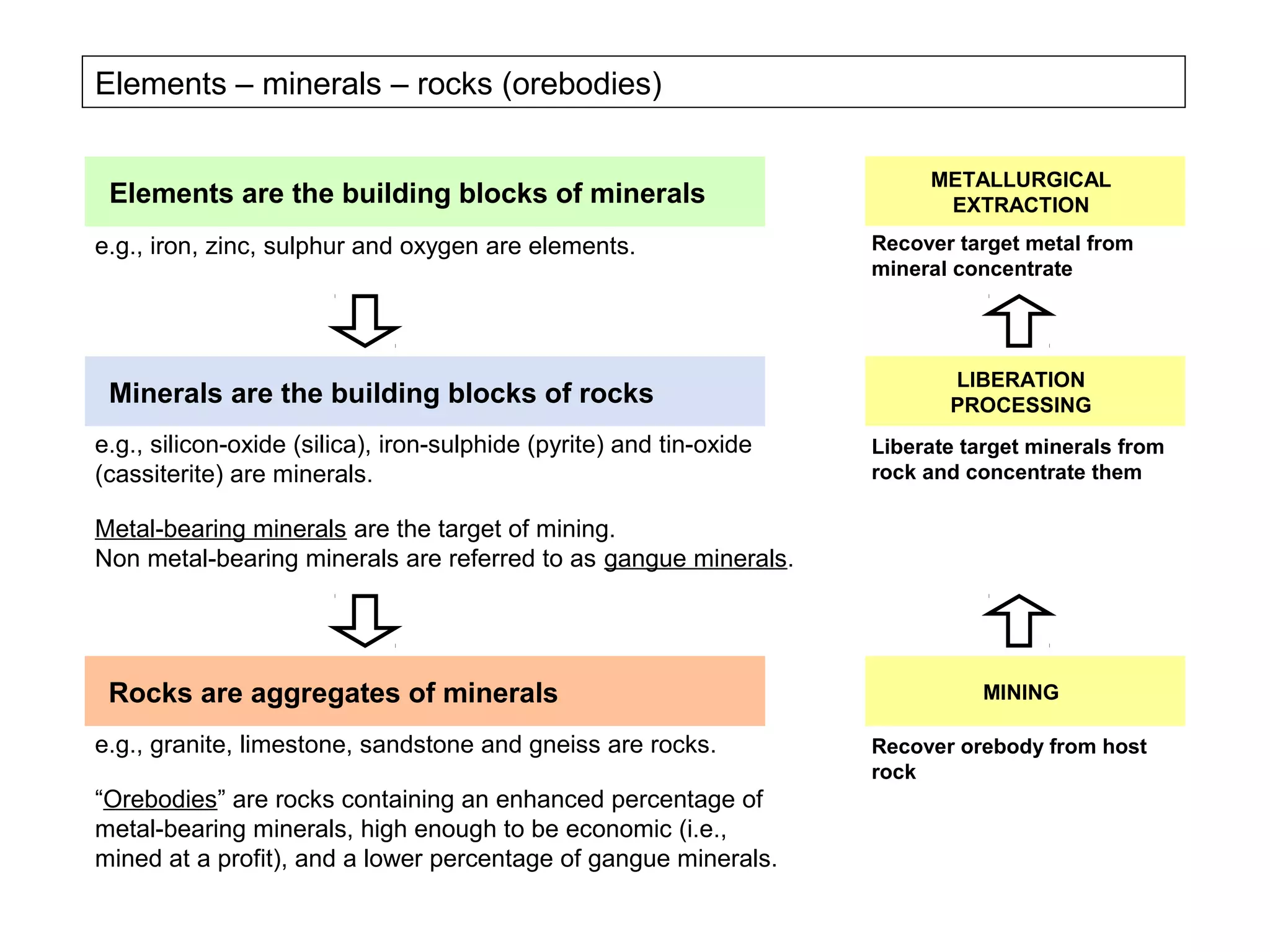
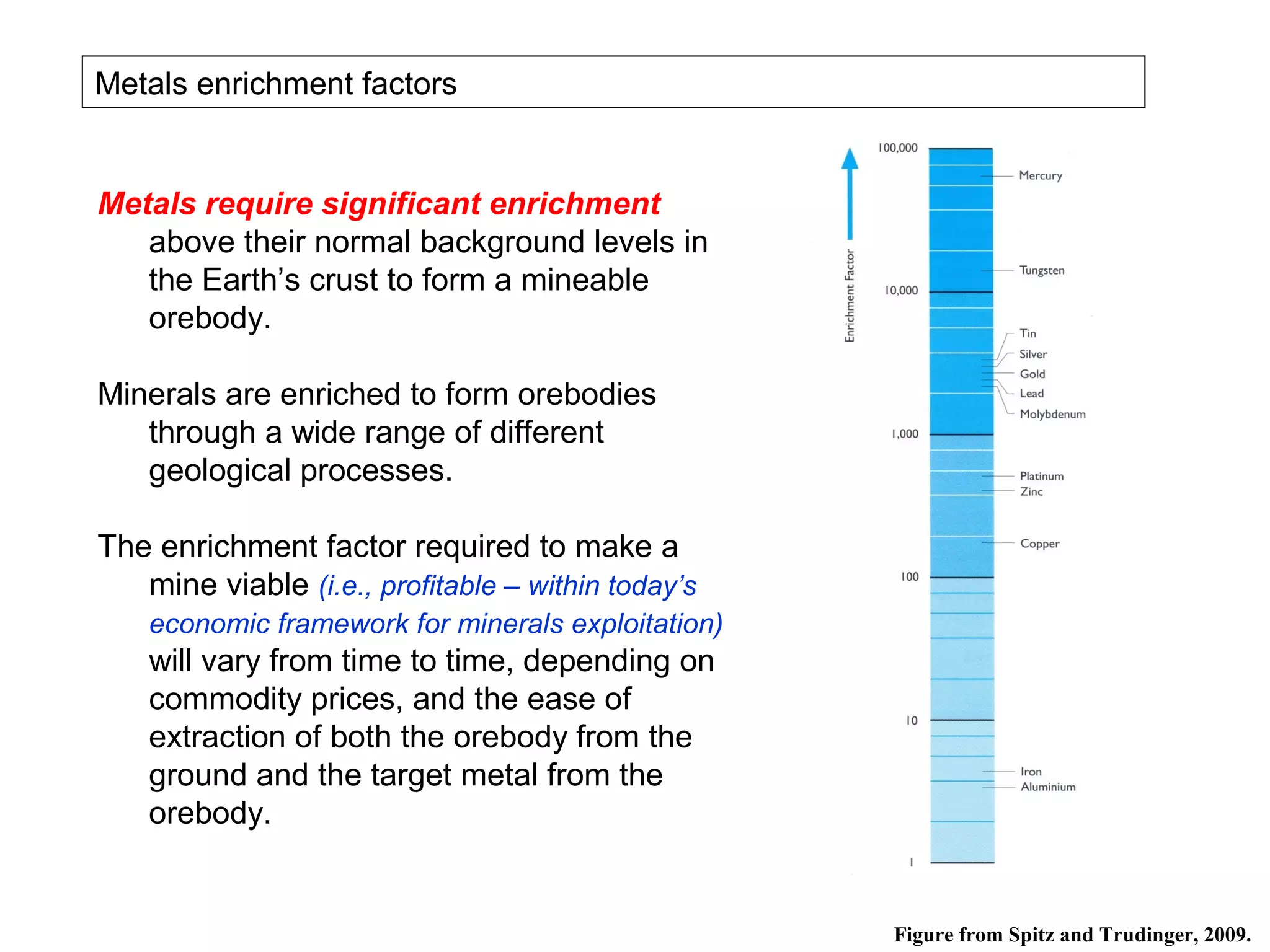
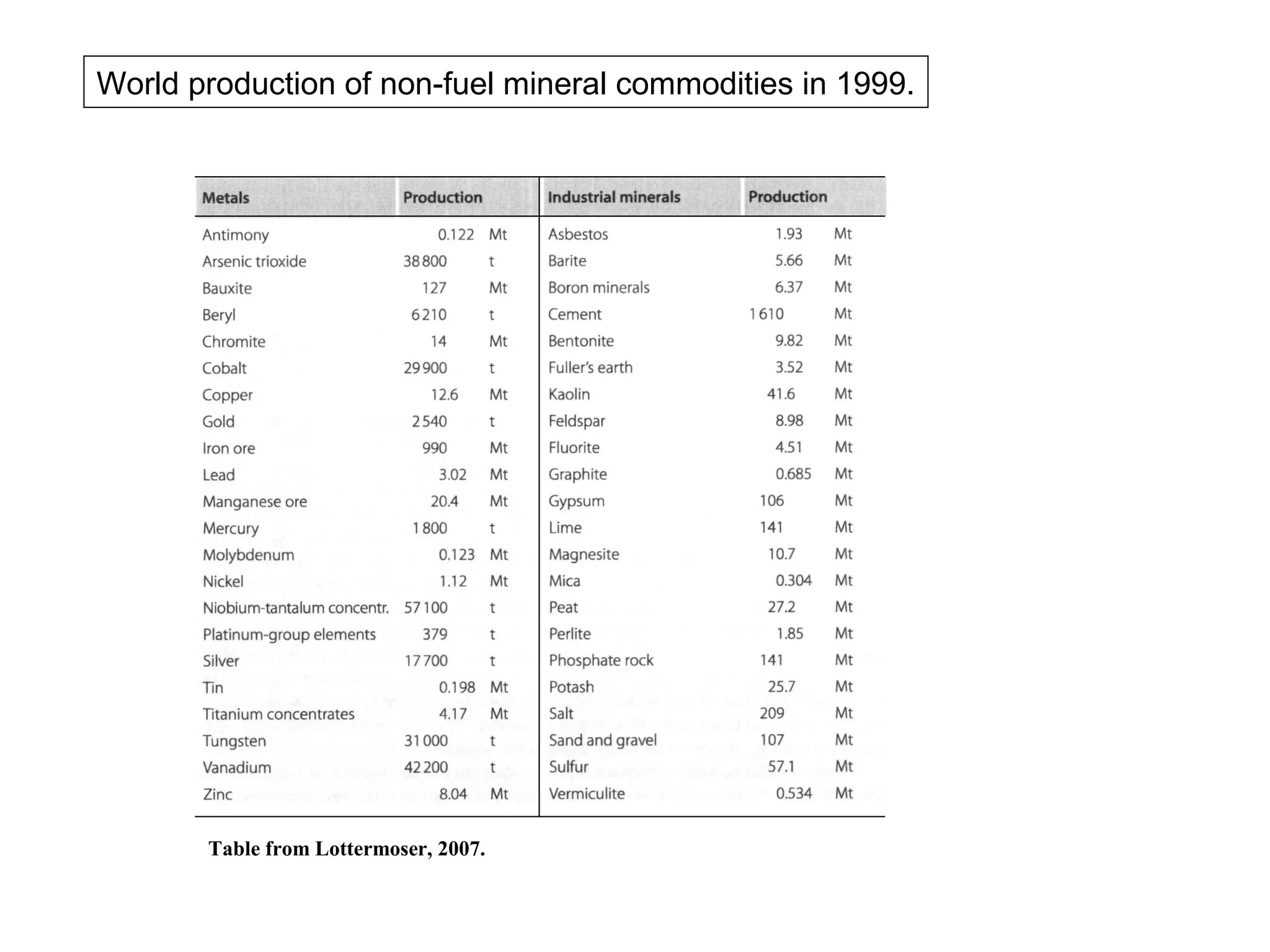
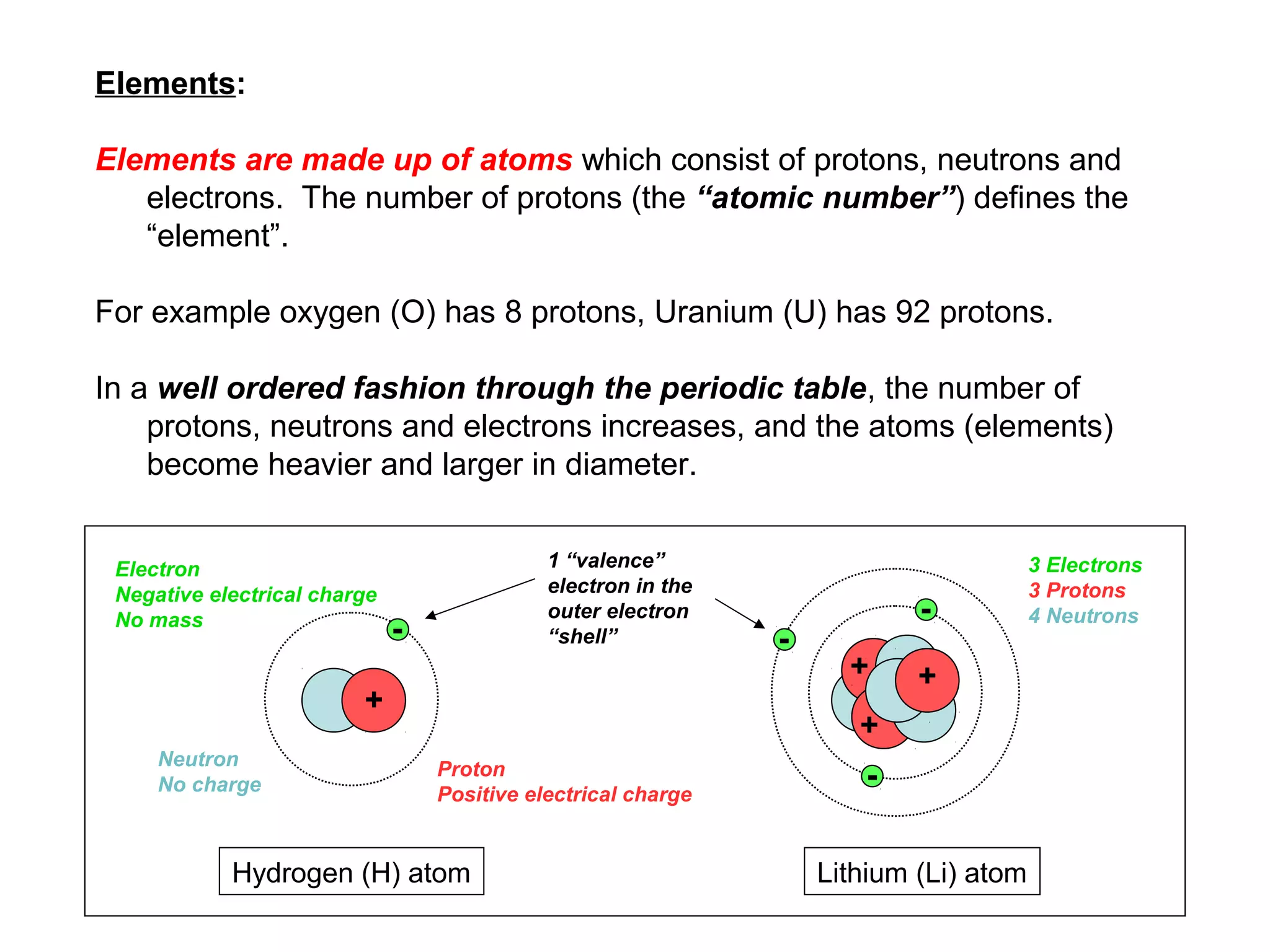
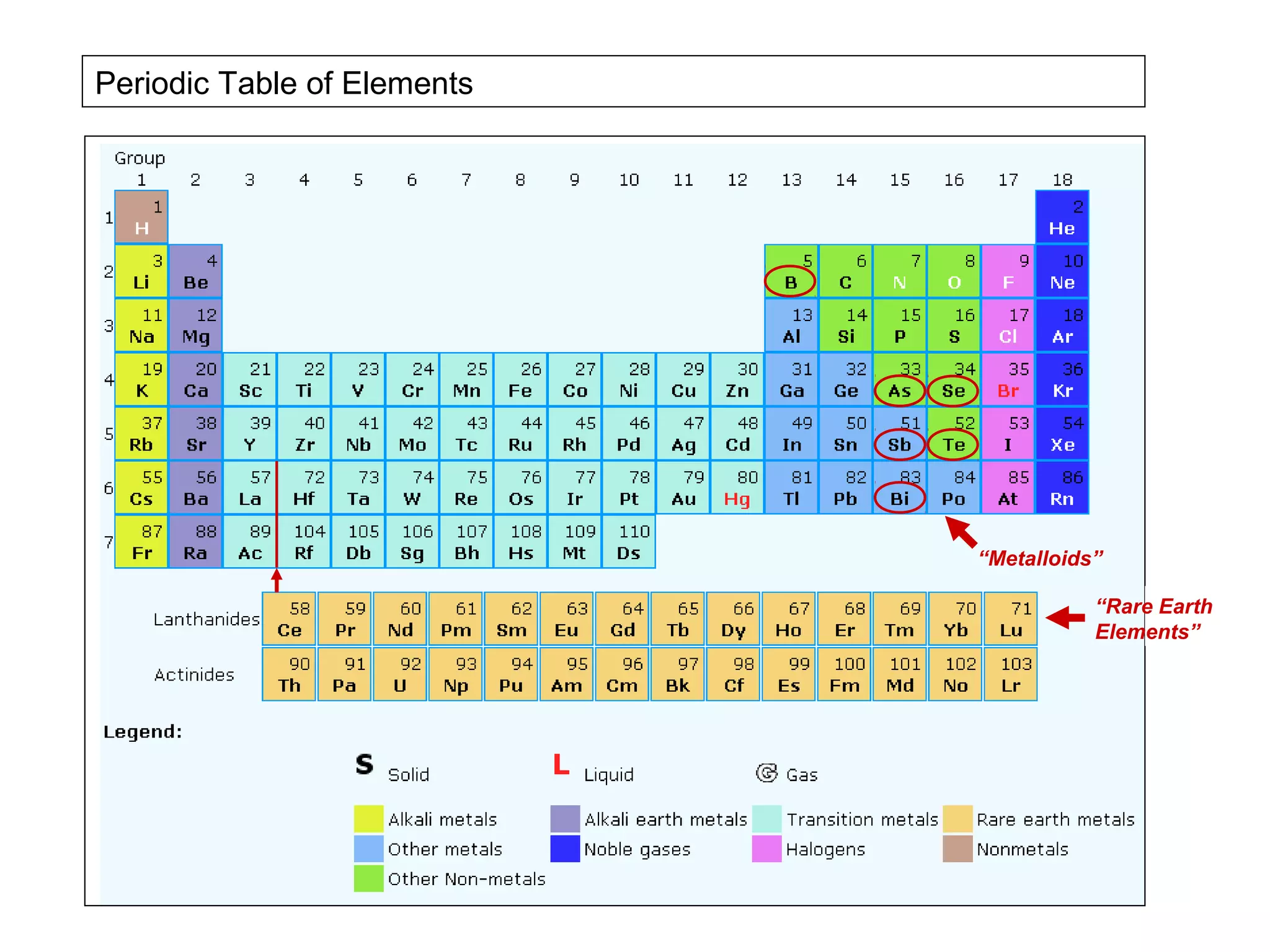
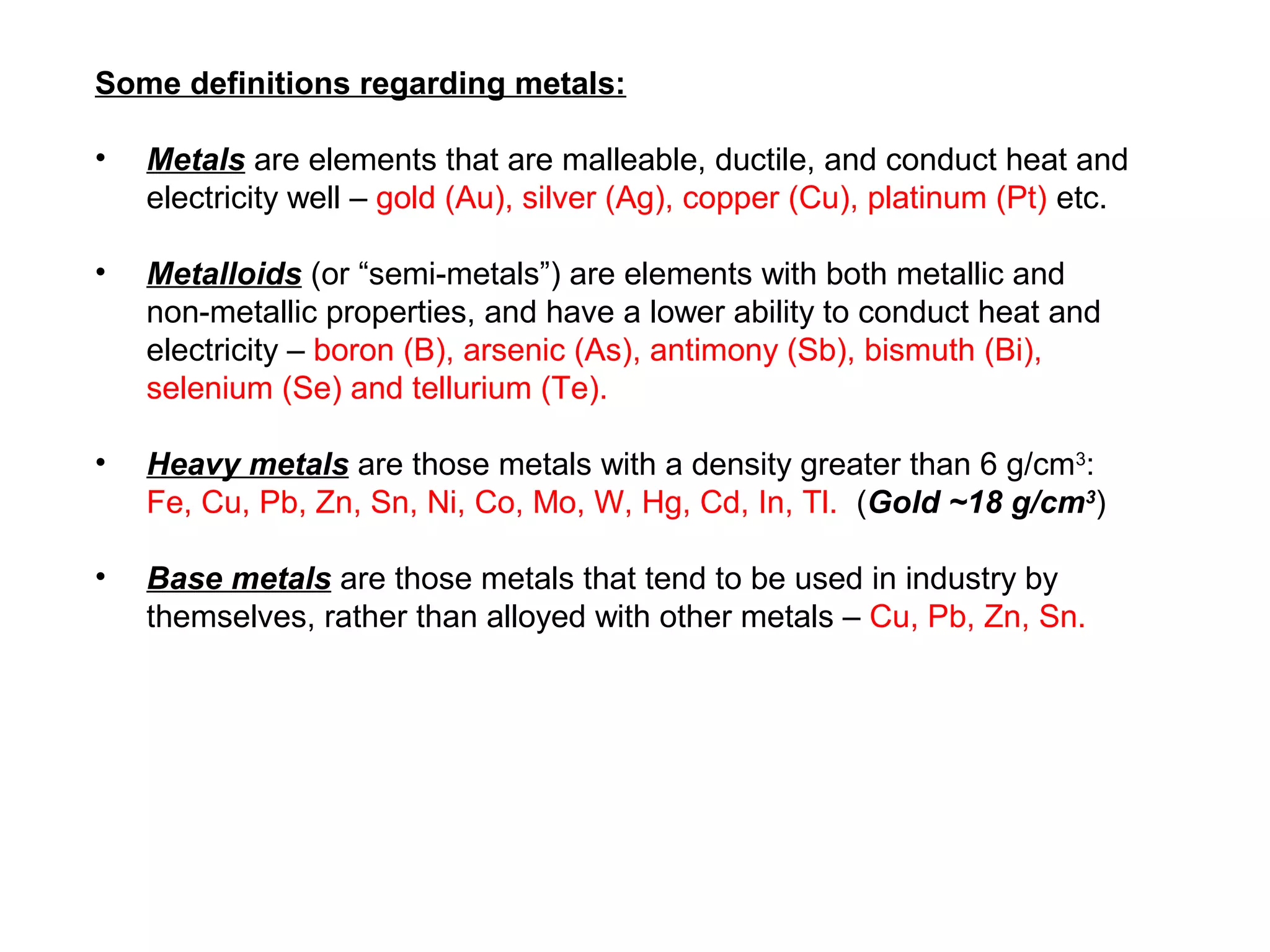
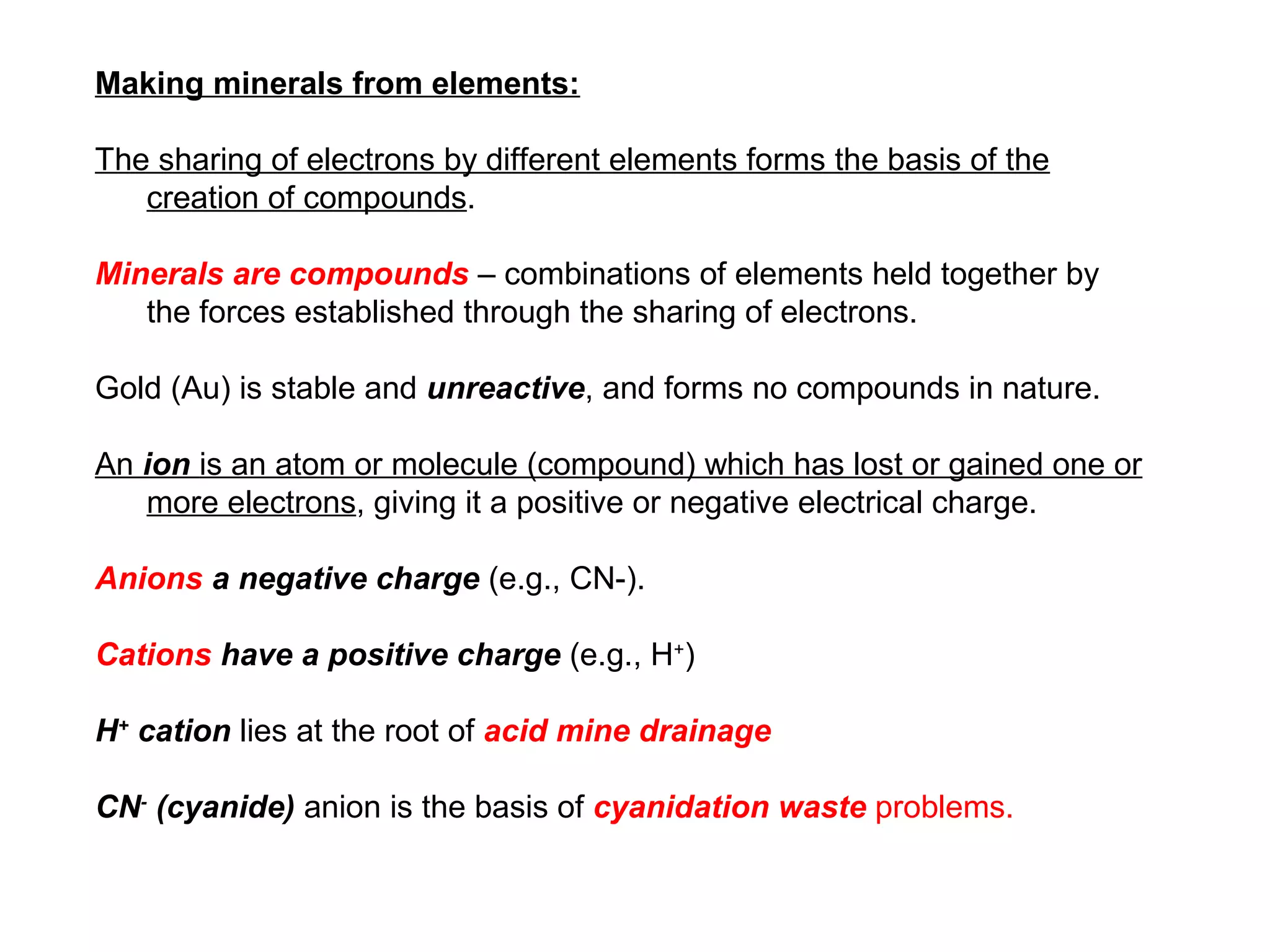
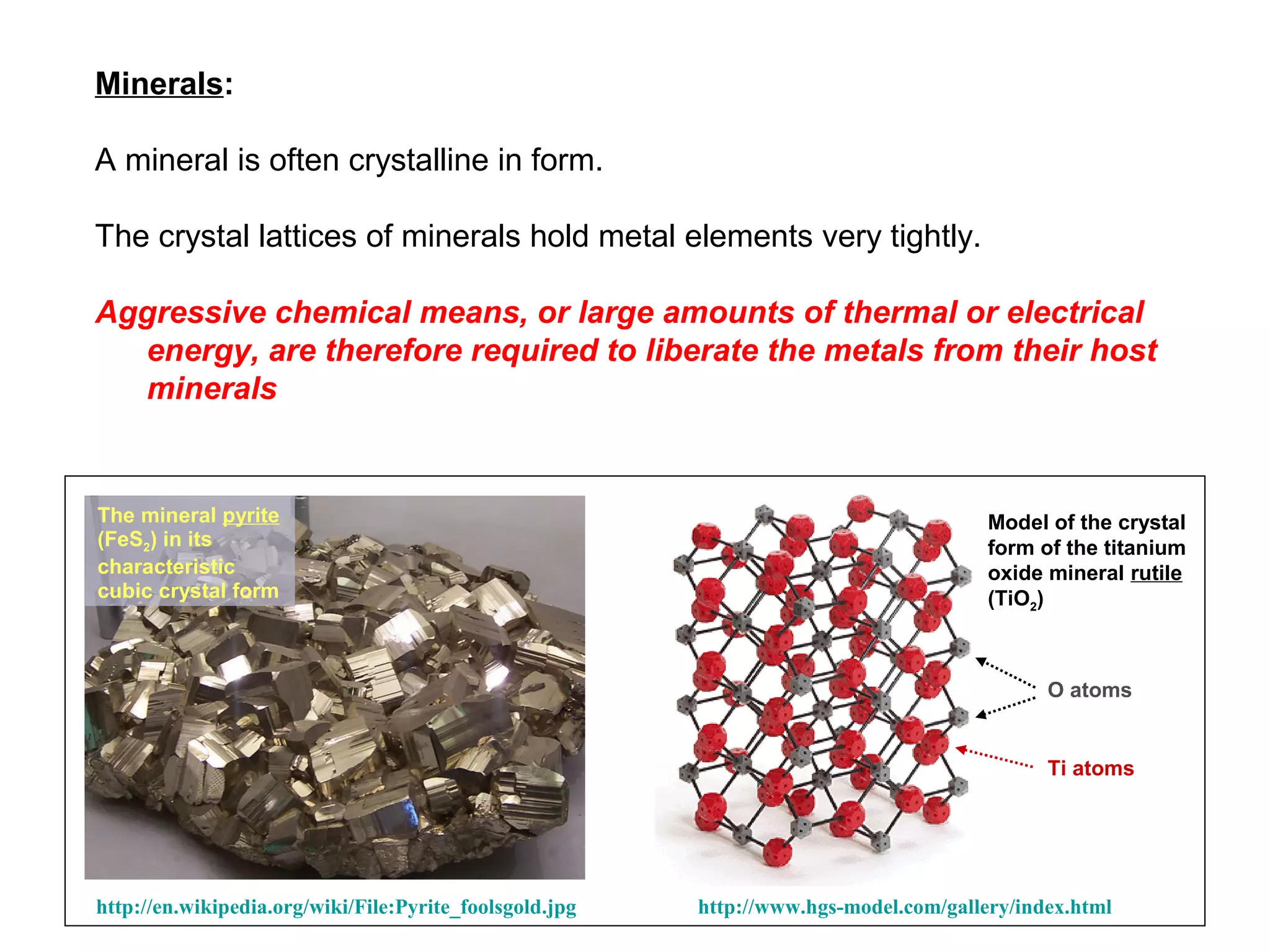
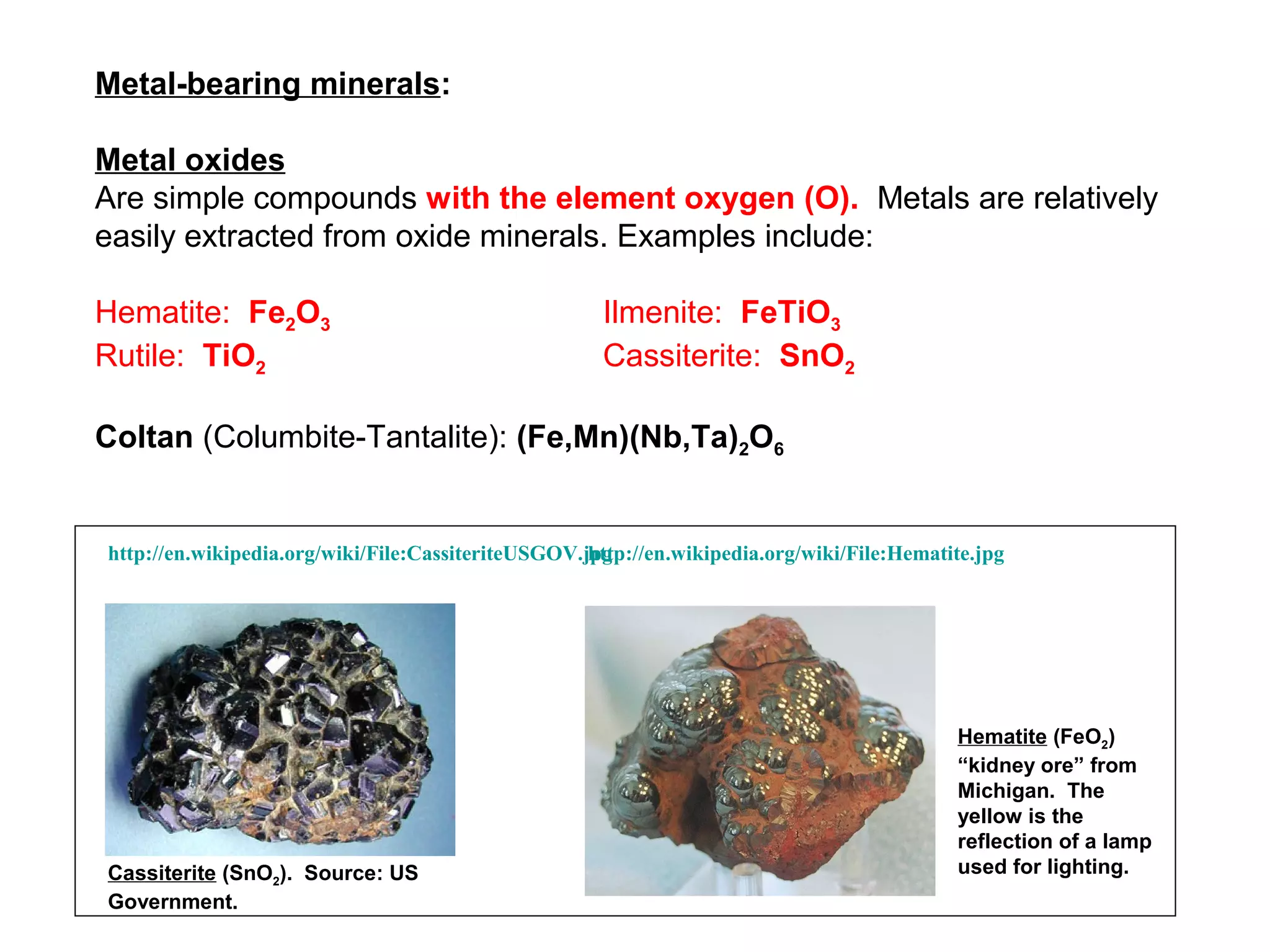
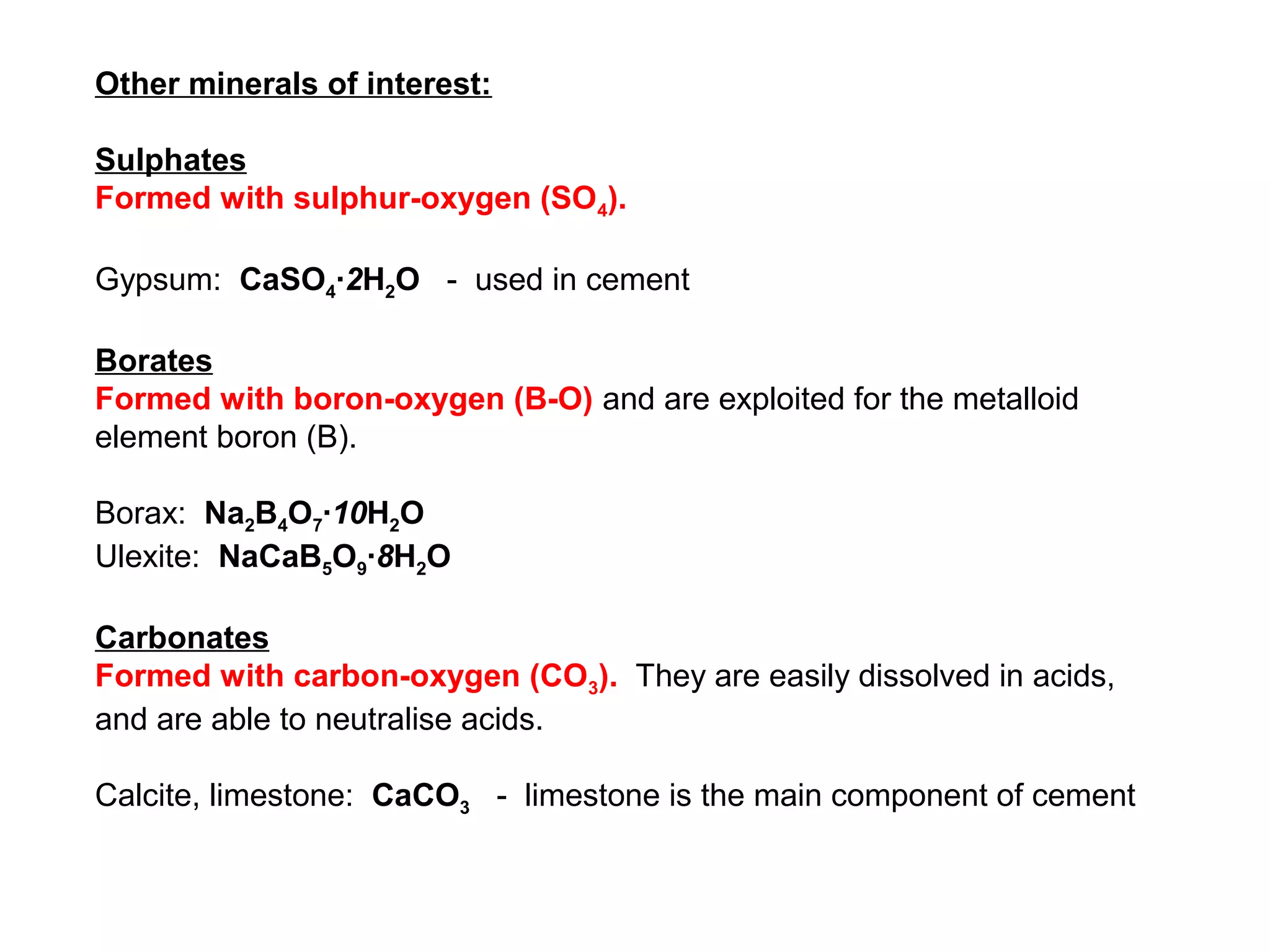
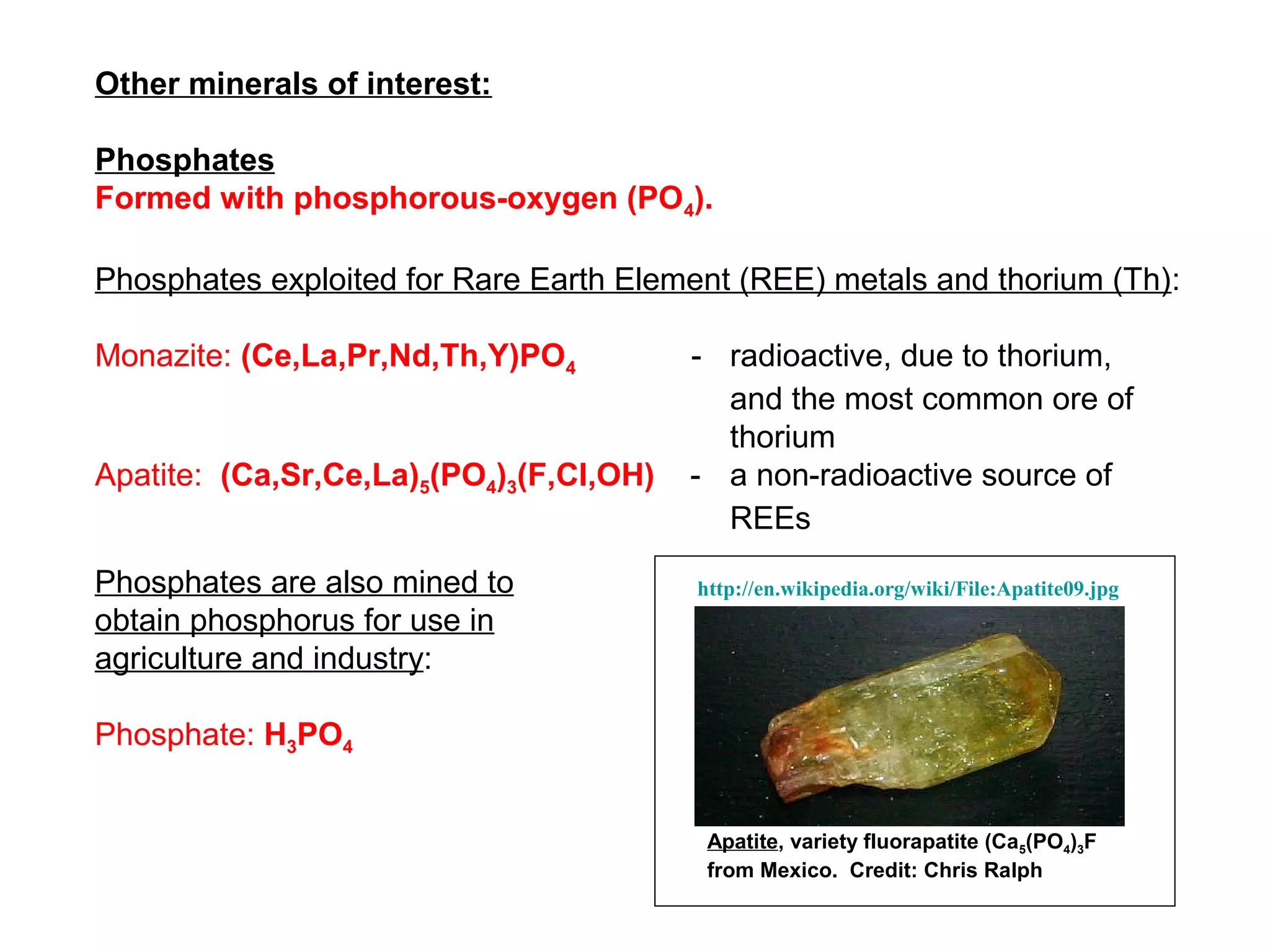
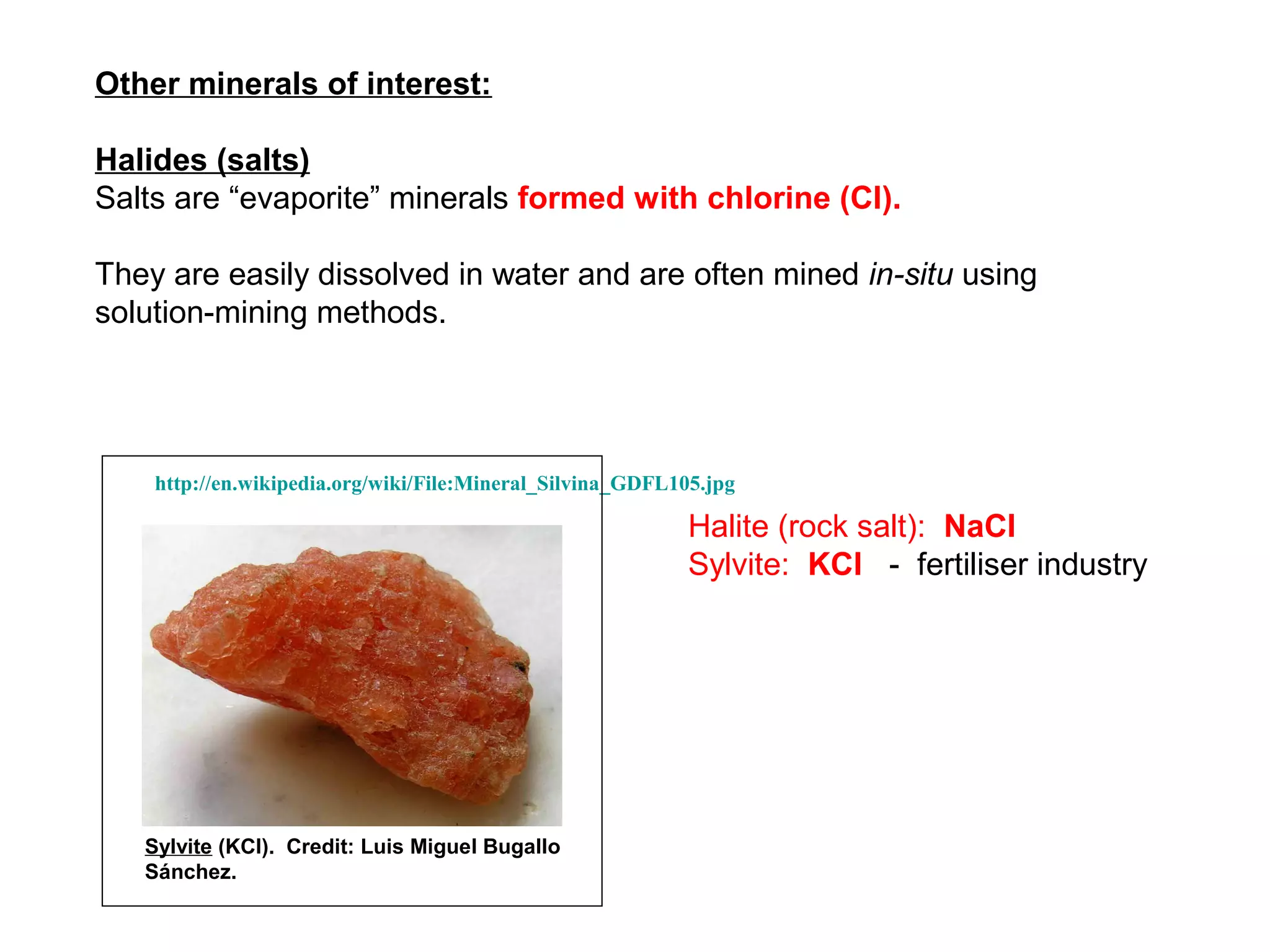
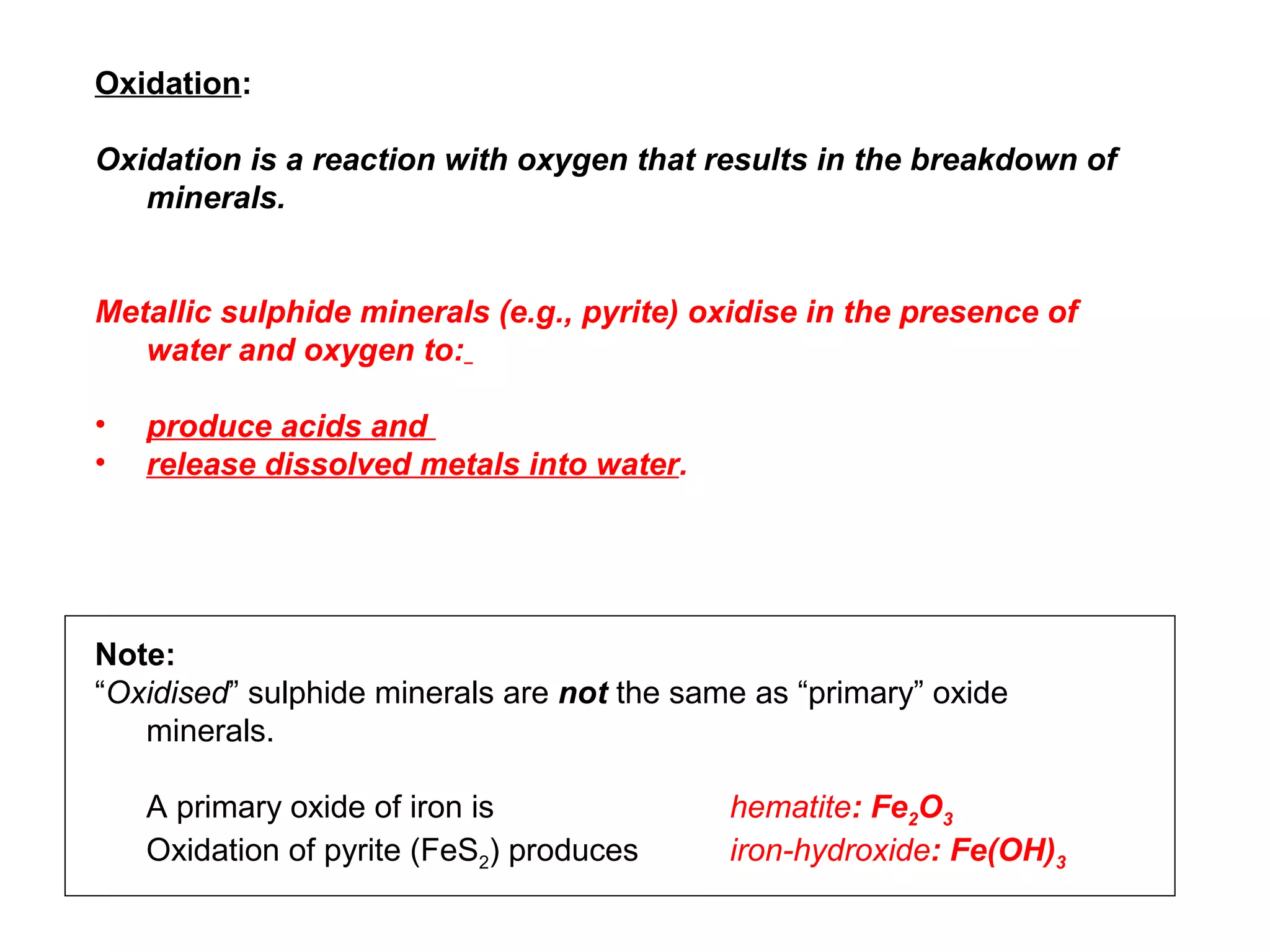
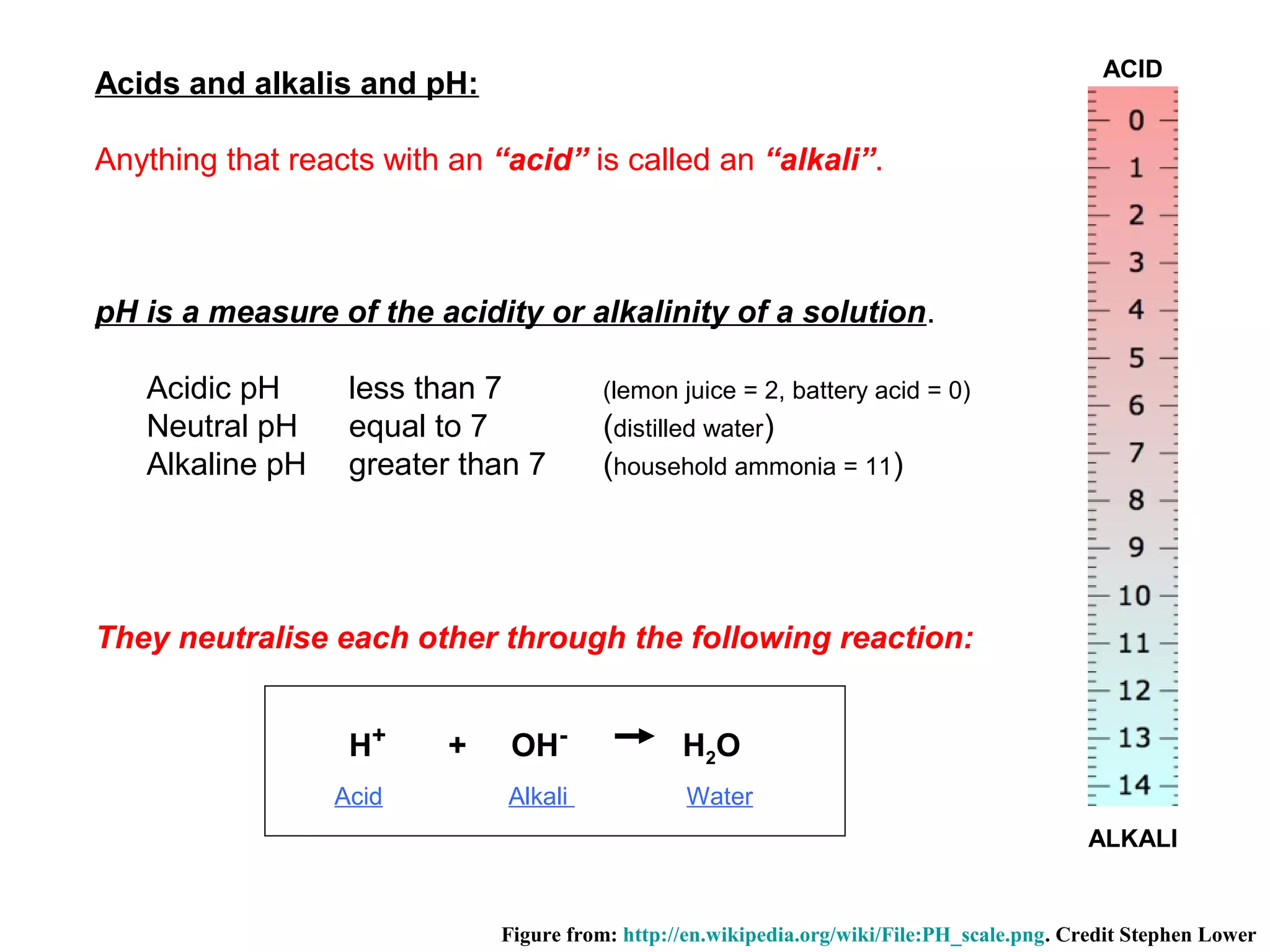
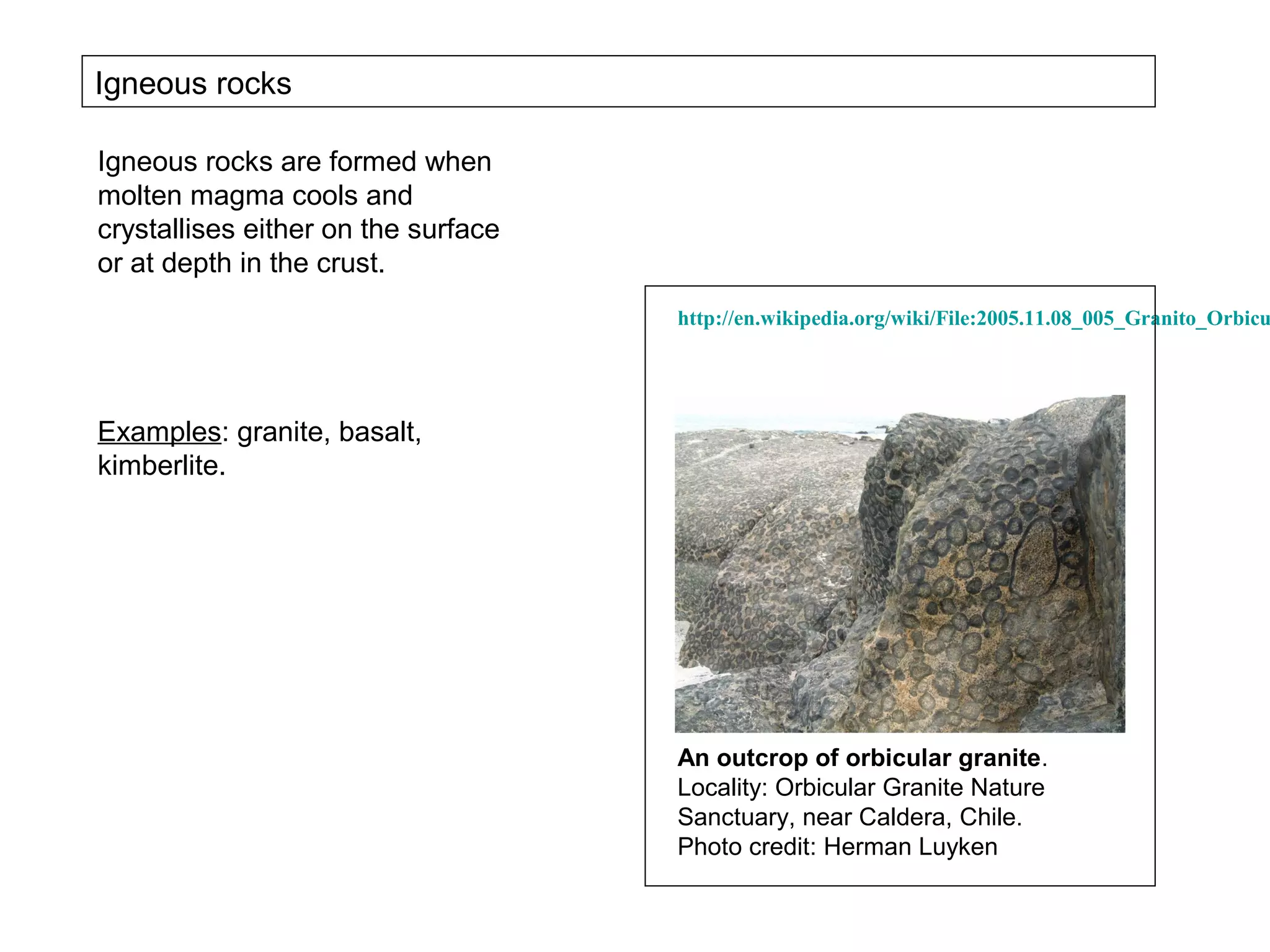
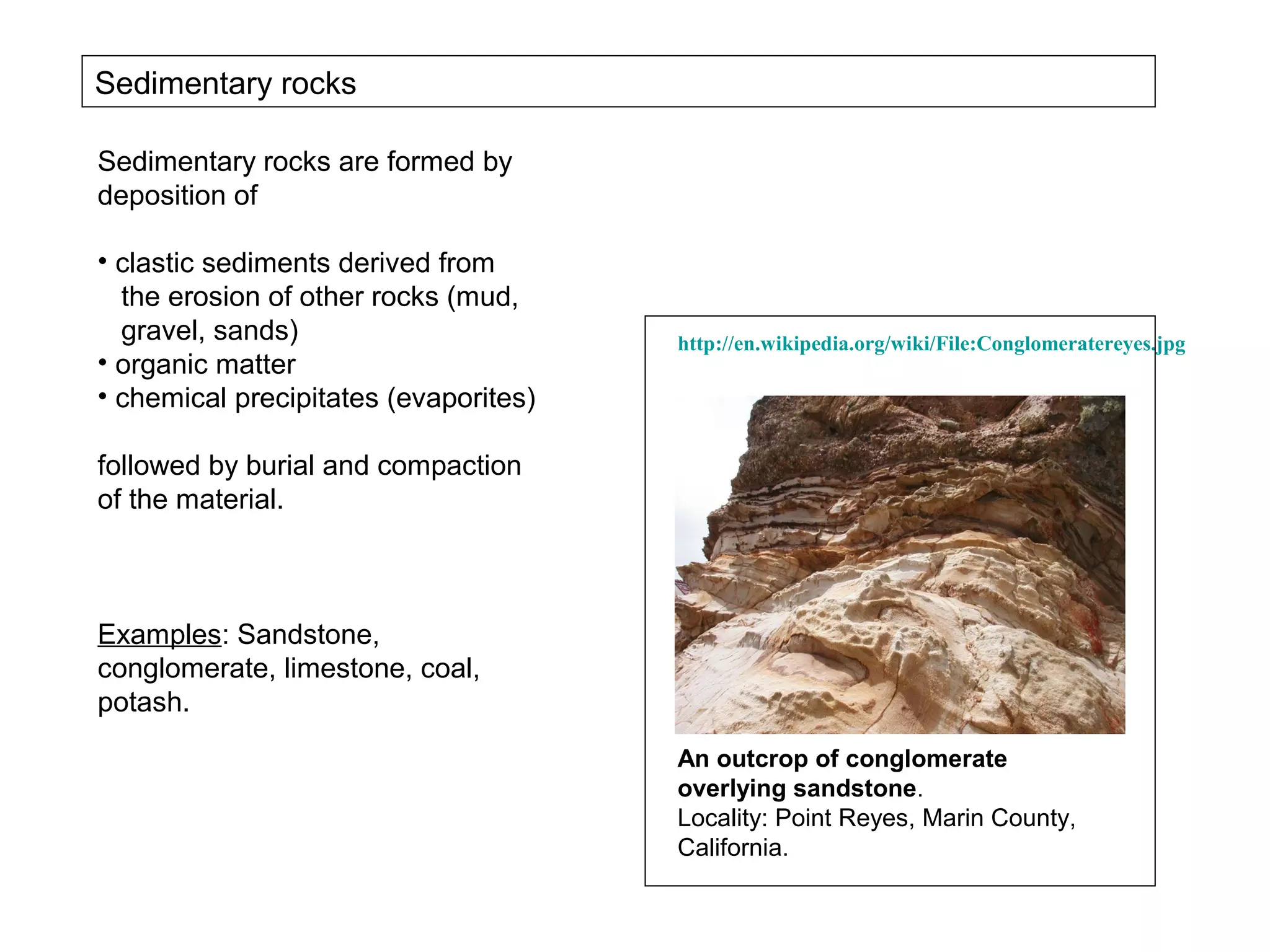
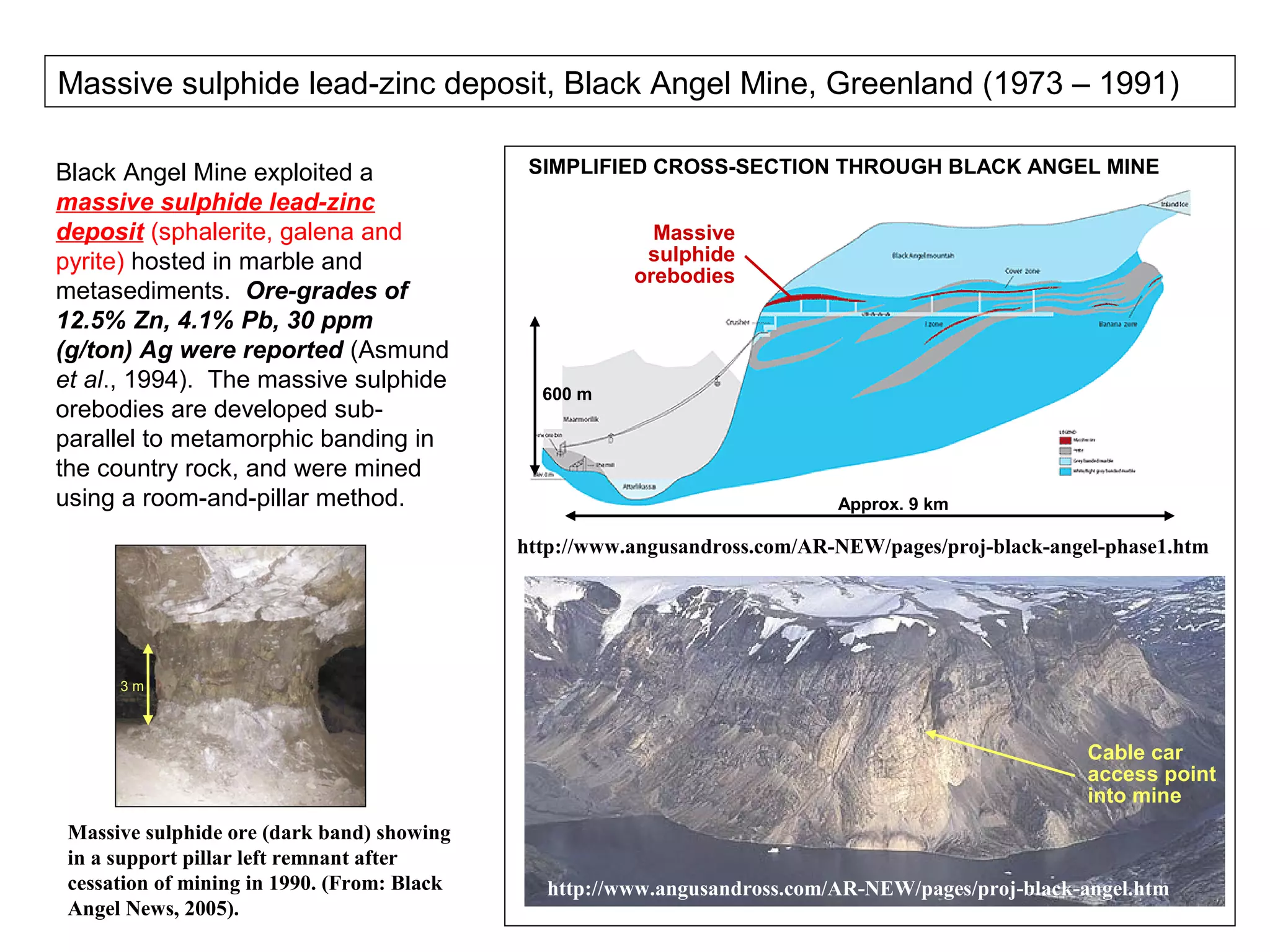
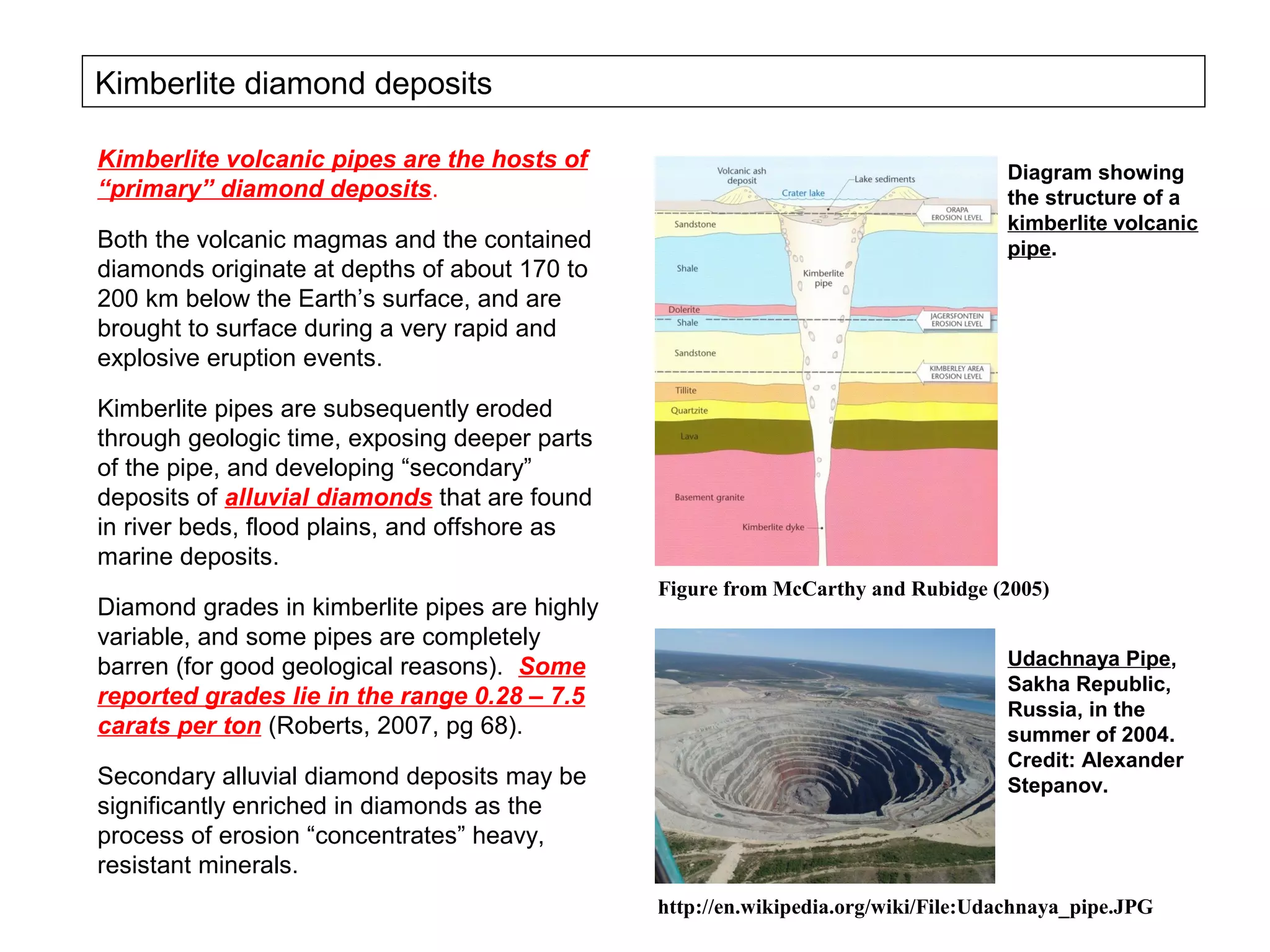
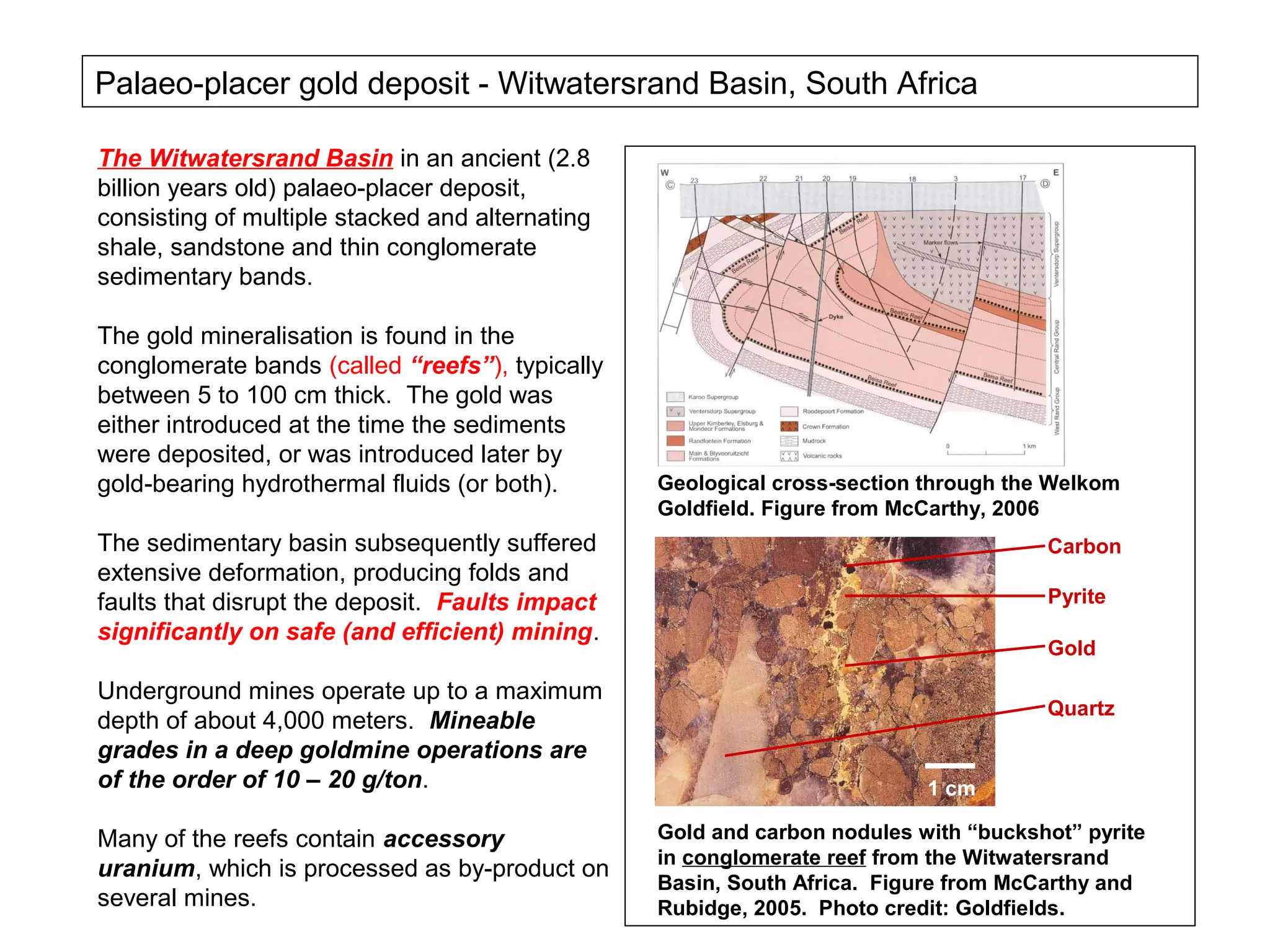
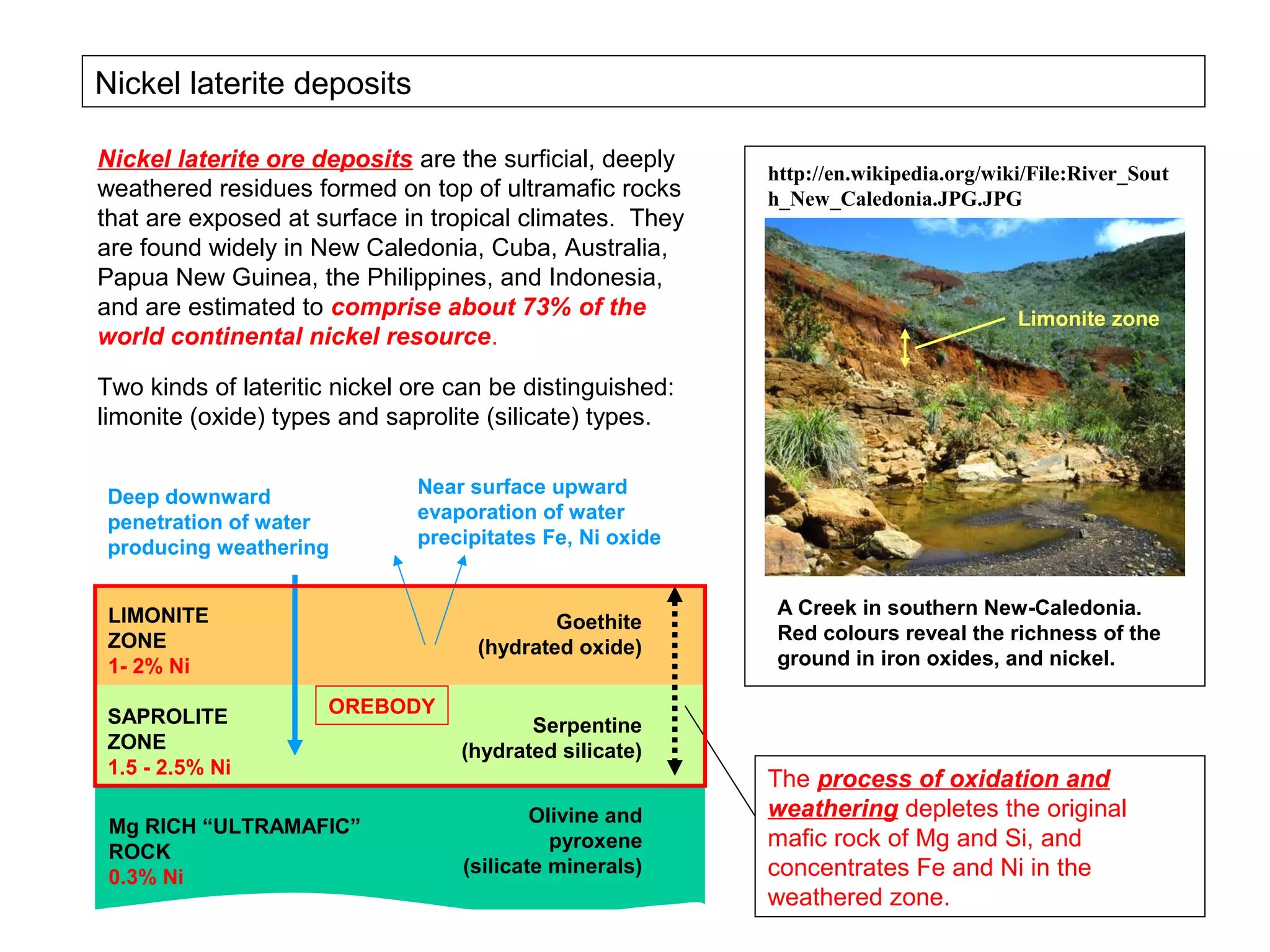
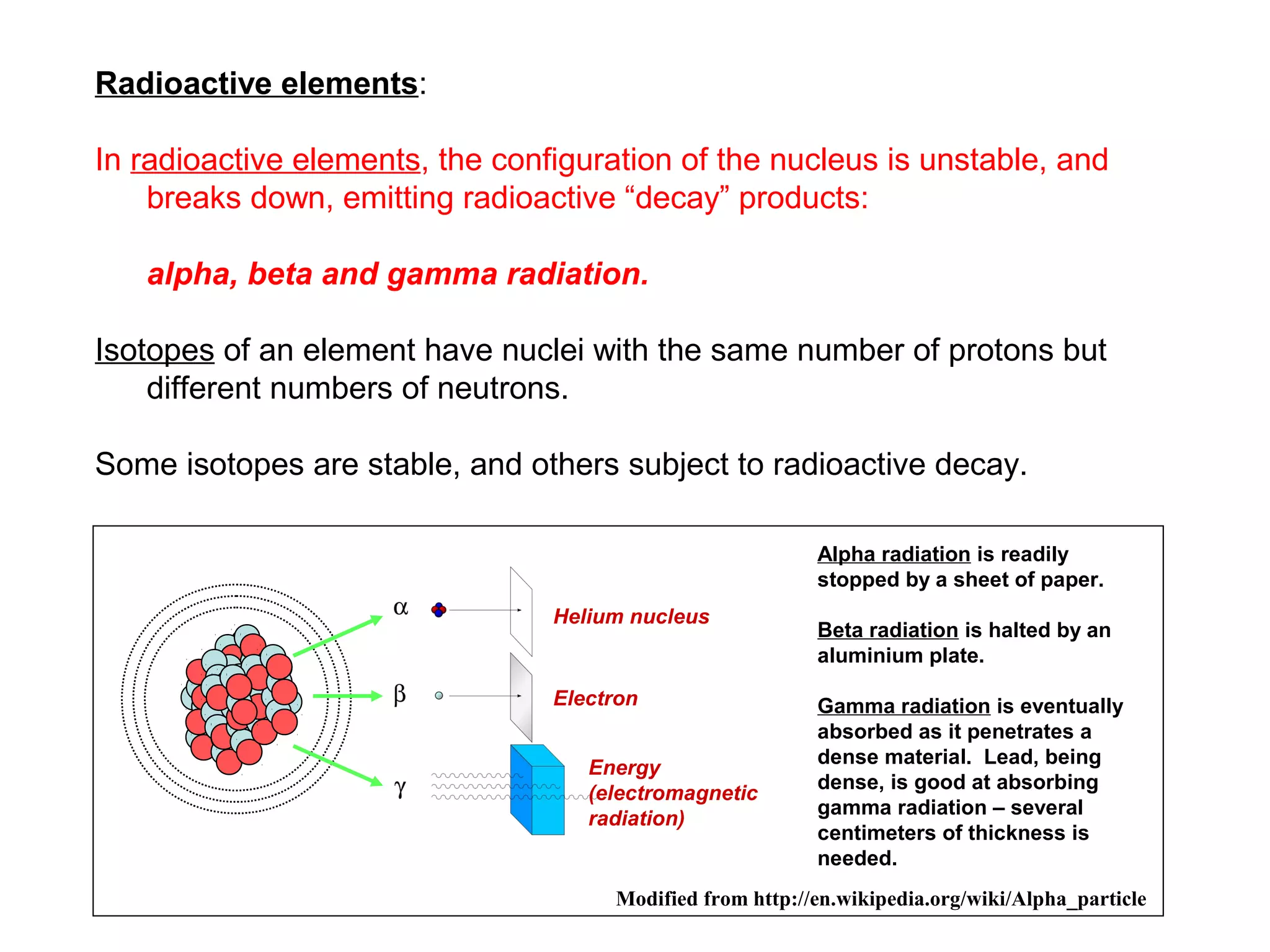
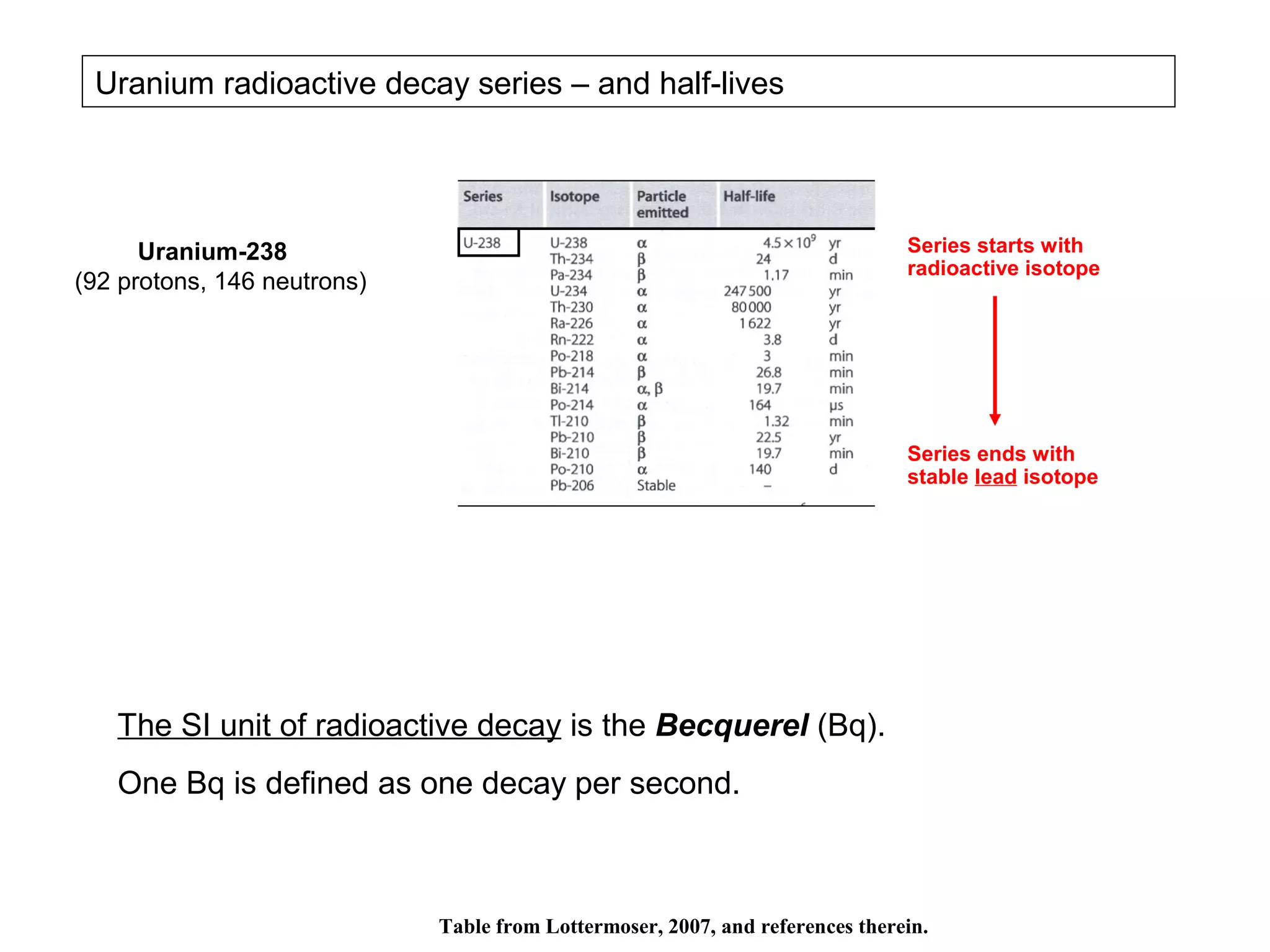
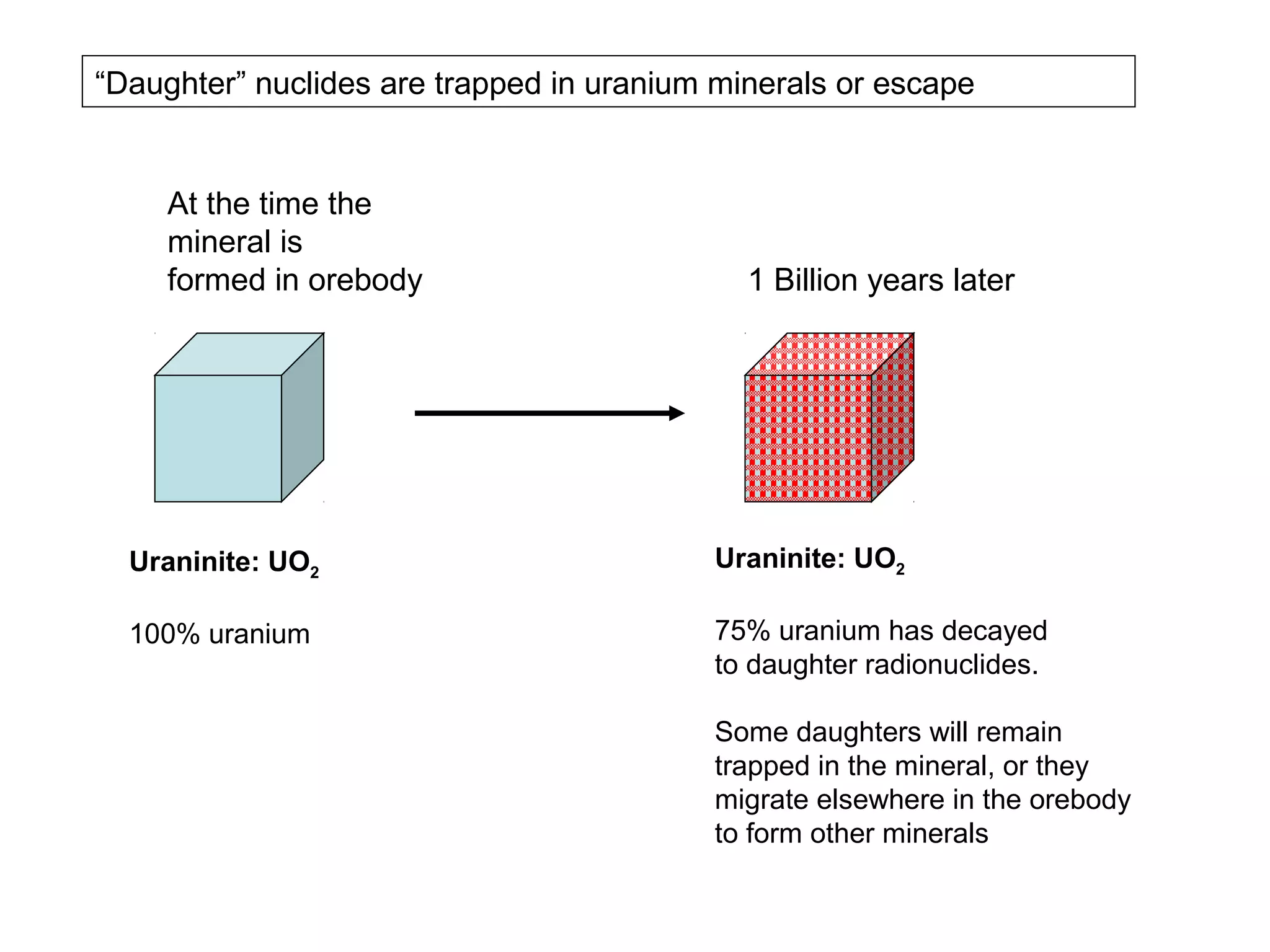
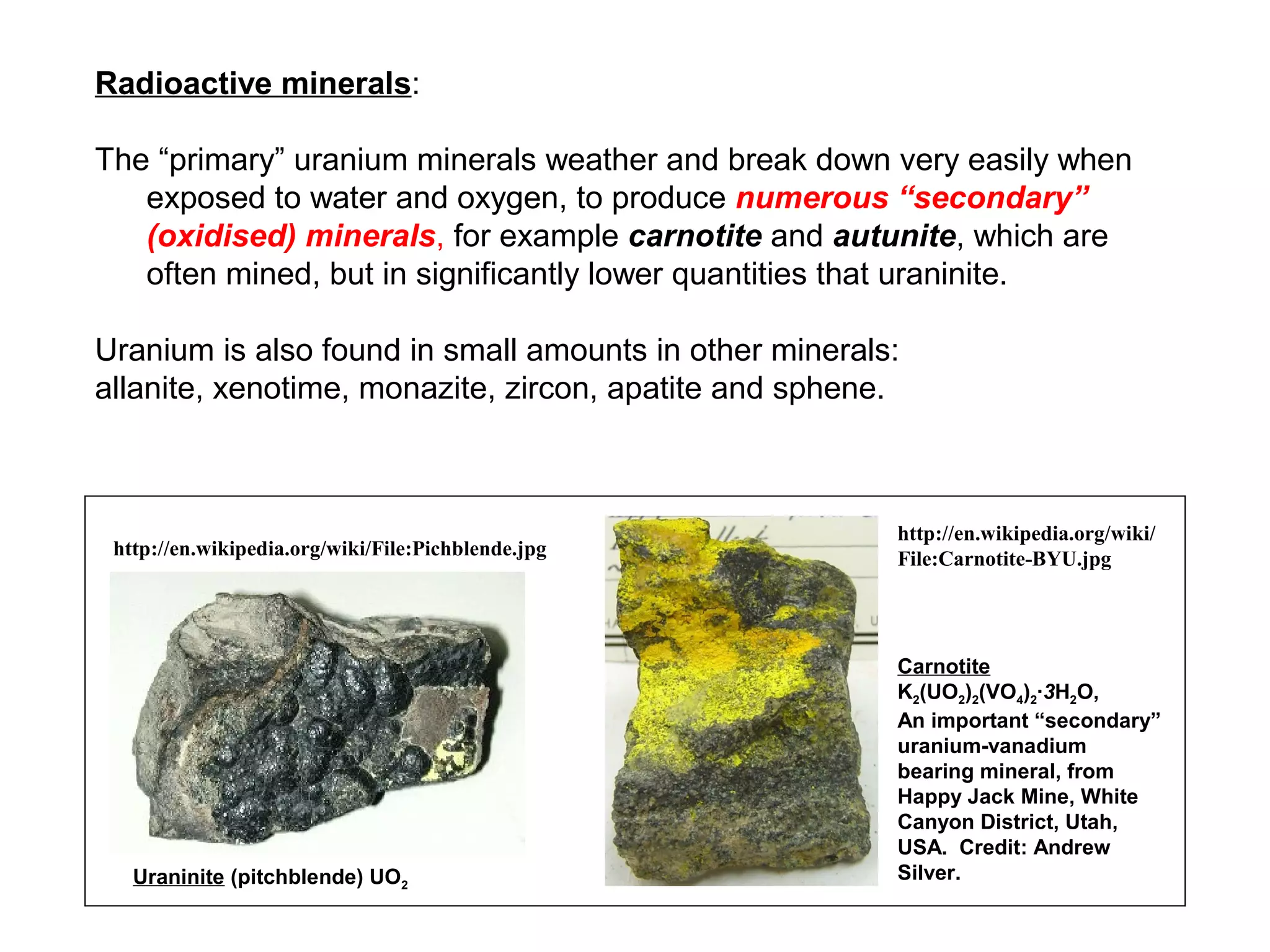
The document provides an outline for a series of lectures on metals, minerals, mining and environmental problems. It discusses various topics including ore mineralogy, mining methods, ore processing, waste management, and environmental and social concerns. Specific problems examined include surface subsidence from underground mining, rockbursts, tailings dam failures, cyanidation wastes, radioactive wastes, and acid mine drainage. The document also provides background information on elements, minerals, rock types, and ore deposit geology.