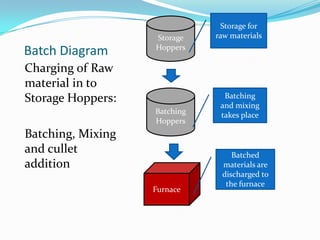
Glass is a hard, brittle material made by melting together silica sand, soda ash, limestone and other ingredients at high temperatures. It can be formed into different shapes through processes like glassblowing, pressing or drawing and is then annealed to increase its strength. Common types of glass include soda-lime glass for windows and containers, lead crystal for tableware, Pyrex for labware and heat resistance, and optical glass for lenses. Glass fibers are used for insulation, reinforcement and other applications. Modern glass production involves batching raw materials, melting in furnaces, forming the glass, annealing and inspection.