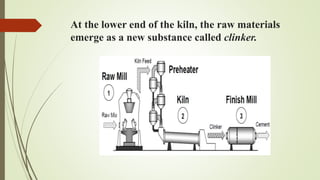
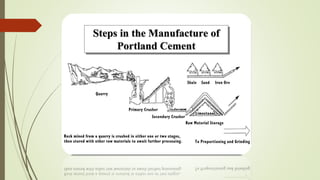
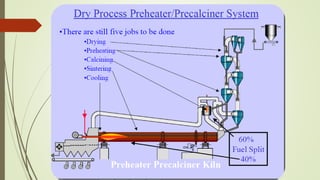
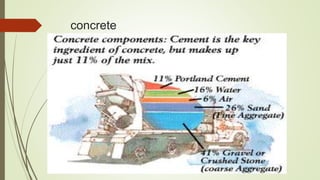
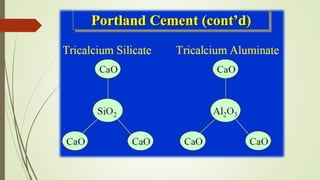
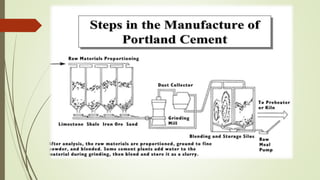
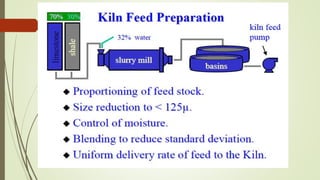
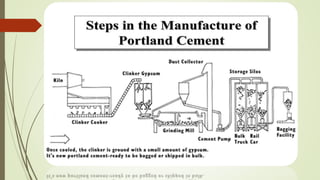
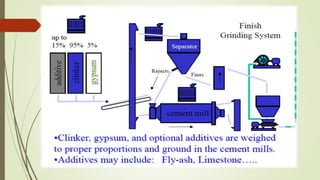
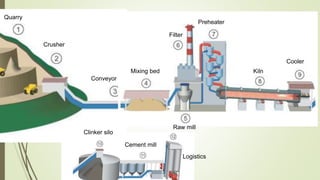
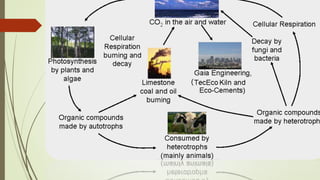
This document discusses the cement manufacturing process. It begins with the history of cement, which has been made since Roman times but has been refined over time. There are four main types of cement. The production process consists of three steps - raw material processing, clinker burning, and finish grinding. The raw material and clinker burning steps can be wet or dry processes. The dry process dries and heats materials directly while the wet process adds water. Portland cement is the most common type and is made by heating limestone and clay. The production process involves quarrying, crushing, mixing, heating in a kiln, cooling, and grinding. Emissions from manufacturing like NOx, CO2 and dust must be controlled to reduce