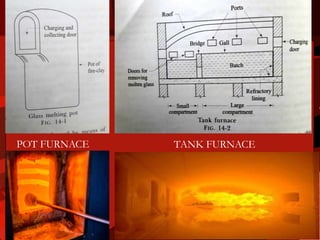
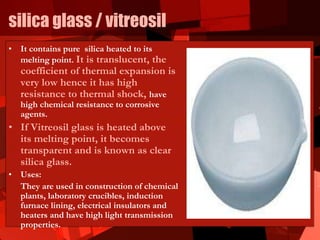
Glass is an amorphous solid made through the melting and cooling of raw materials such as silica sand, soda ash, limestone, and recycled glass. There are many types of glass with different compositions and properties. Glass is manufactured through mixing raw materials, melting in furnaces, fabrication into desired shapes, and annealing to relieve internal stresses. Common glass products include float glass, fiberglass, safety glass, and specialty glasses used for optical, electrical, and scientific applications.