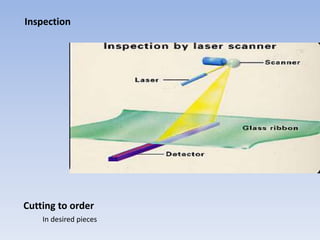
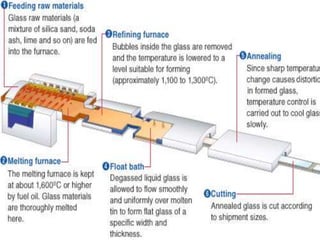
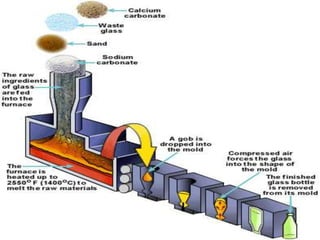
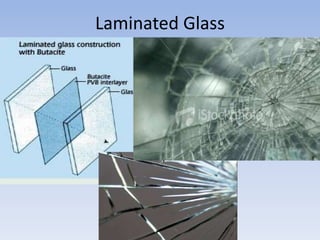
The document provides a comprehensive overview of glass, including its definition, properties, historical development, manufacturing processes, types, and various applications. It highlights the advantages and disadvantages of glass in construction and other uses, emphasizing its utility and challenges. Different types of glass, such as soda-lime, lead, and tempered glass, are detailed along with their specific uses and characteristics.