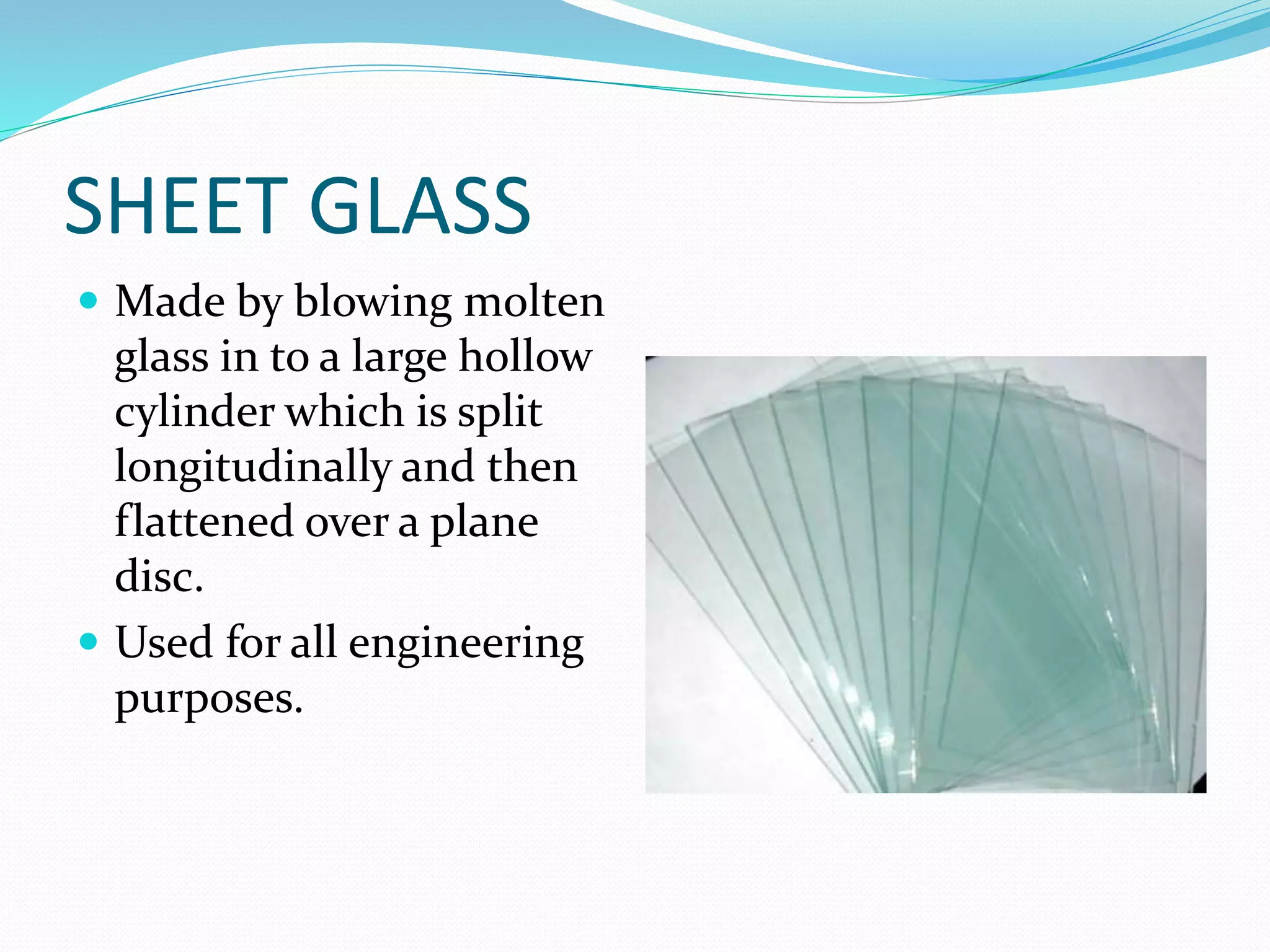
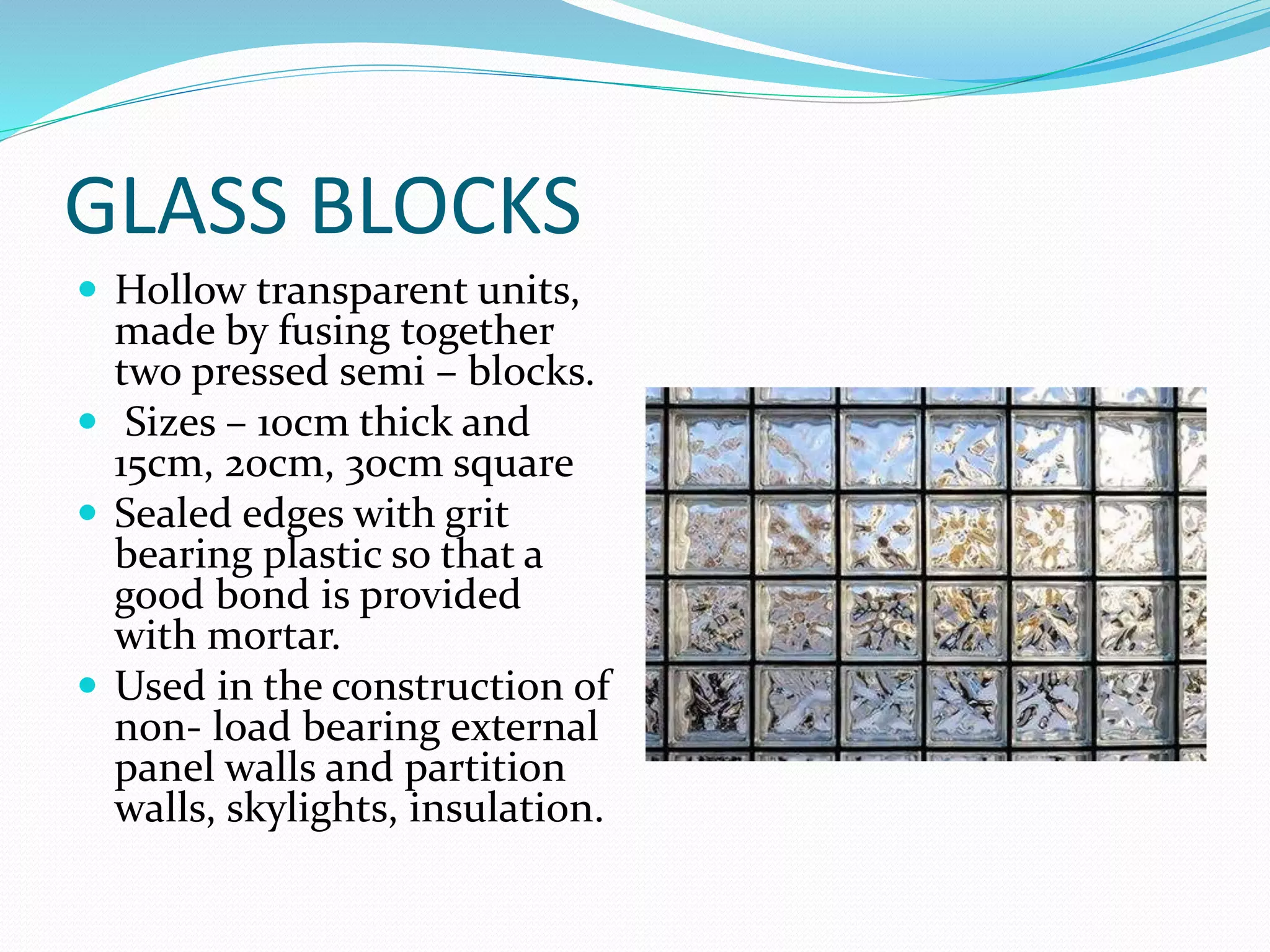
This document discusses the different types and properties of glass. It begins by explaining that glass is made from a mixture of sand and silicates that are melted at high temperatures. It then describes several common types of glass including soda-lime glass, potash-lime glass, potash-lead glass, and coloured glass. It provides details on their compositions and typical uses. The document also outlines specialty glasses such as safety glass, bulletproof glass, insulating glass, and glass wool. It aims to cover the major categories and applications of glass.