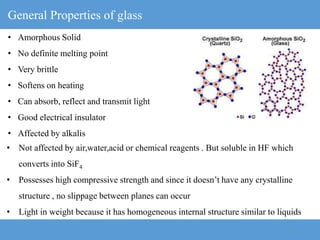
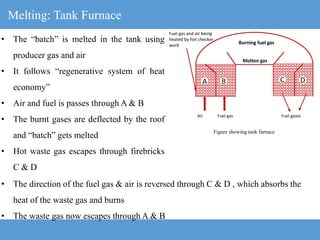
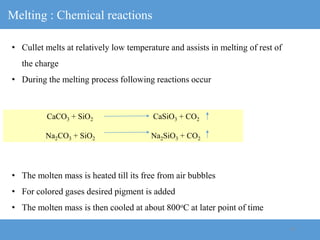
The document provides an overview of engineering ceramics and glasses, including:
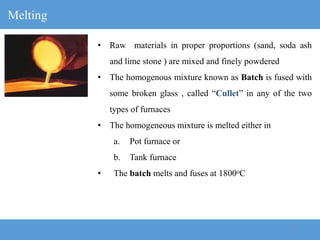
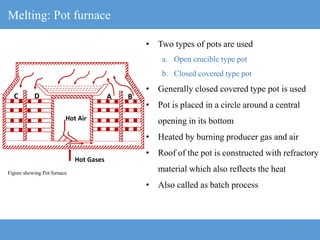
1) The manufacturing process for glass involves melting raw materials at high temperatures, forming and shaping the molten glass, annealing to strengthen it, and finishing.
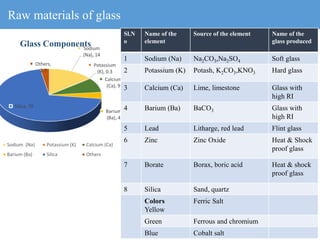
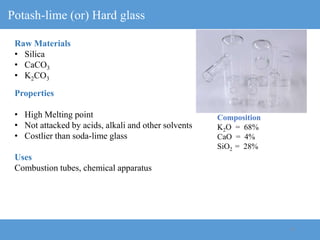
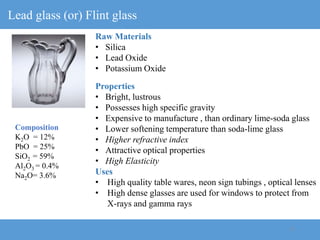
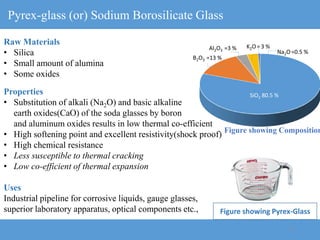
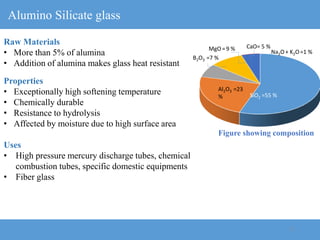
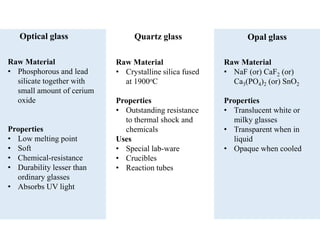
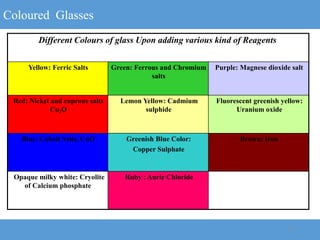
2) Different types of glasses are produced by varying the raw materials, including soda-lime glass, potash-lime glass, lead glass, borosilicate glass, and alumina silicate glass.
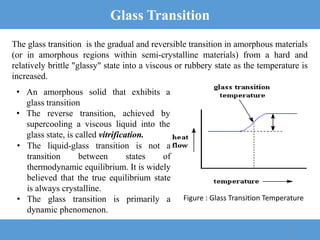
3) The glass transition is the reversible transition of an amorphous material from a hard, brittle state to a rubbery state as temperature increases, and is characterized by the glass transition temperature Tg.