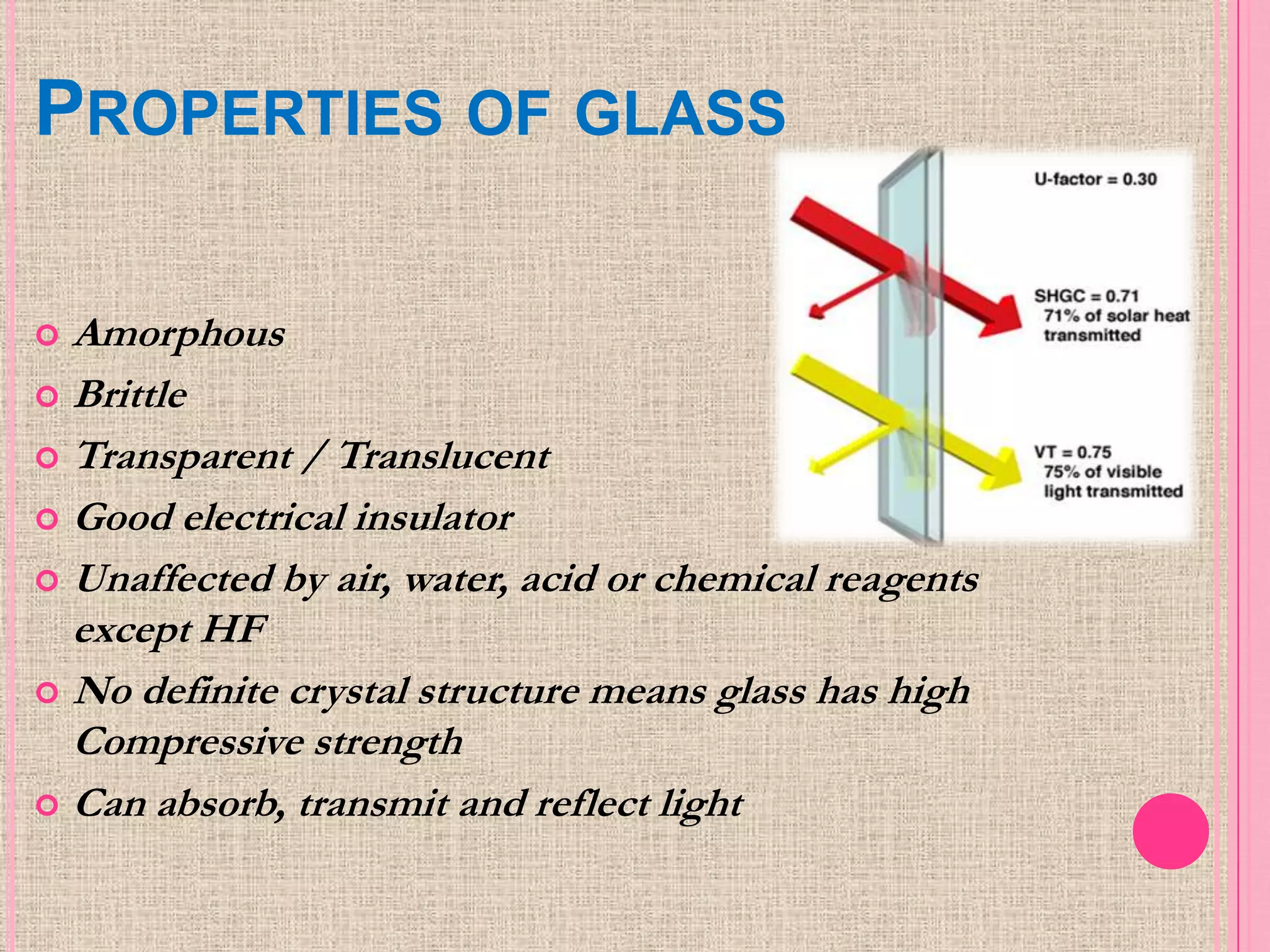
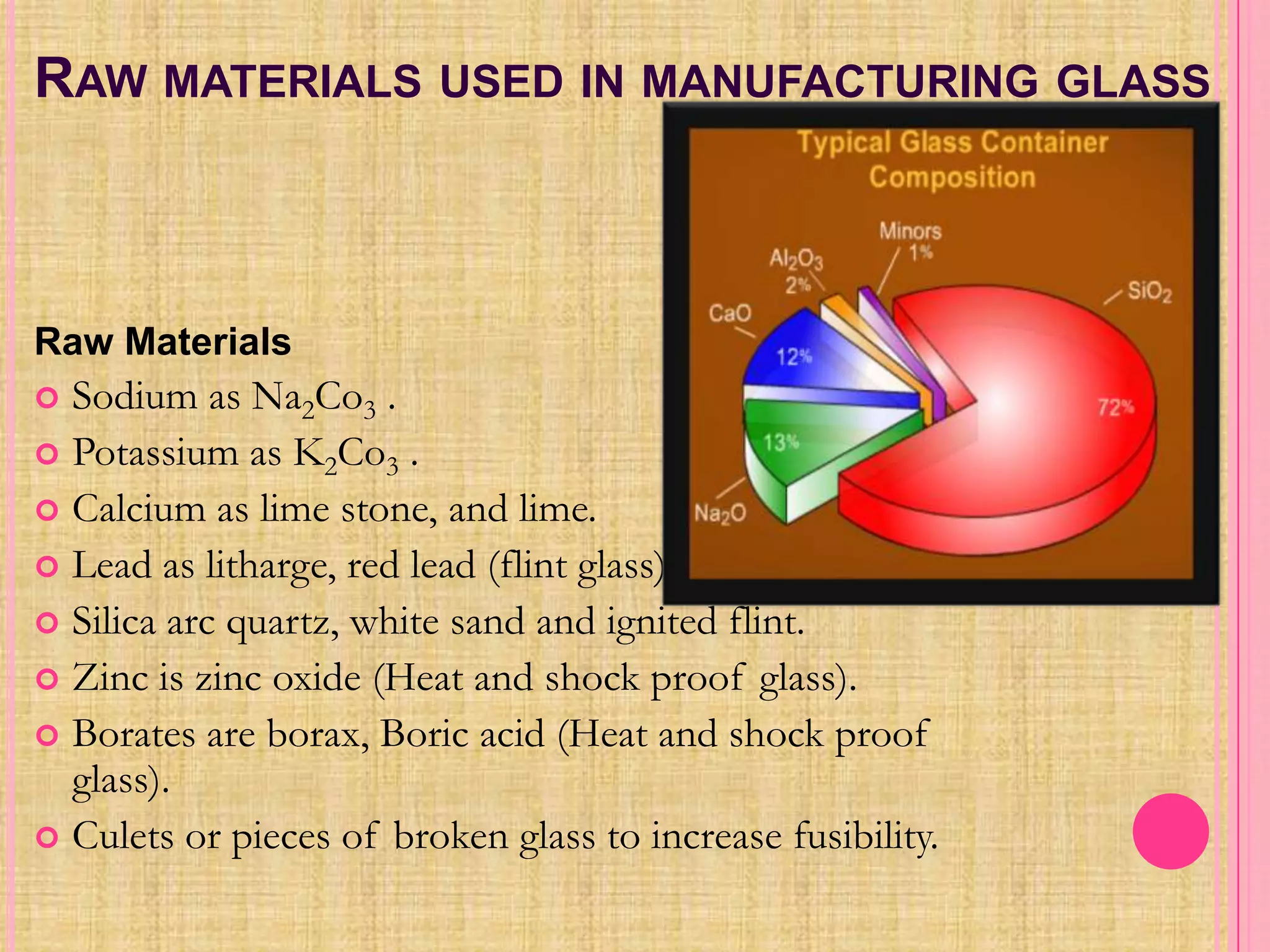
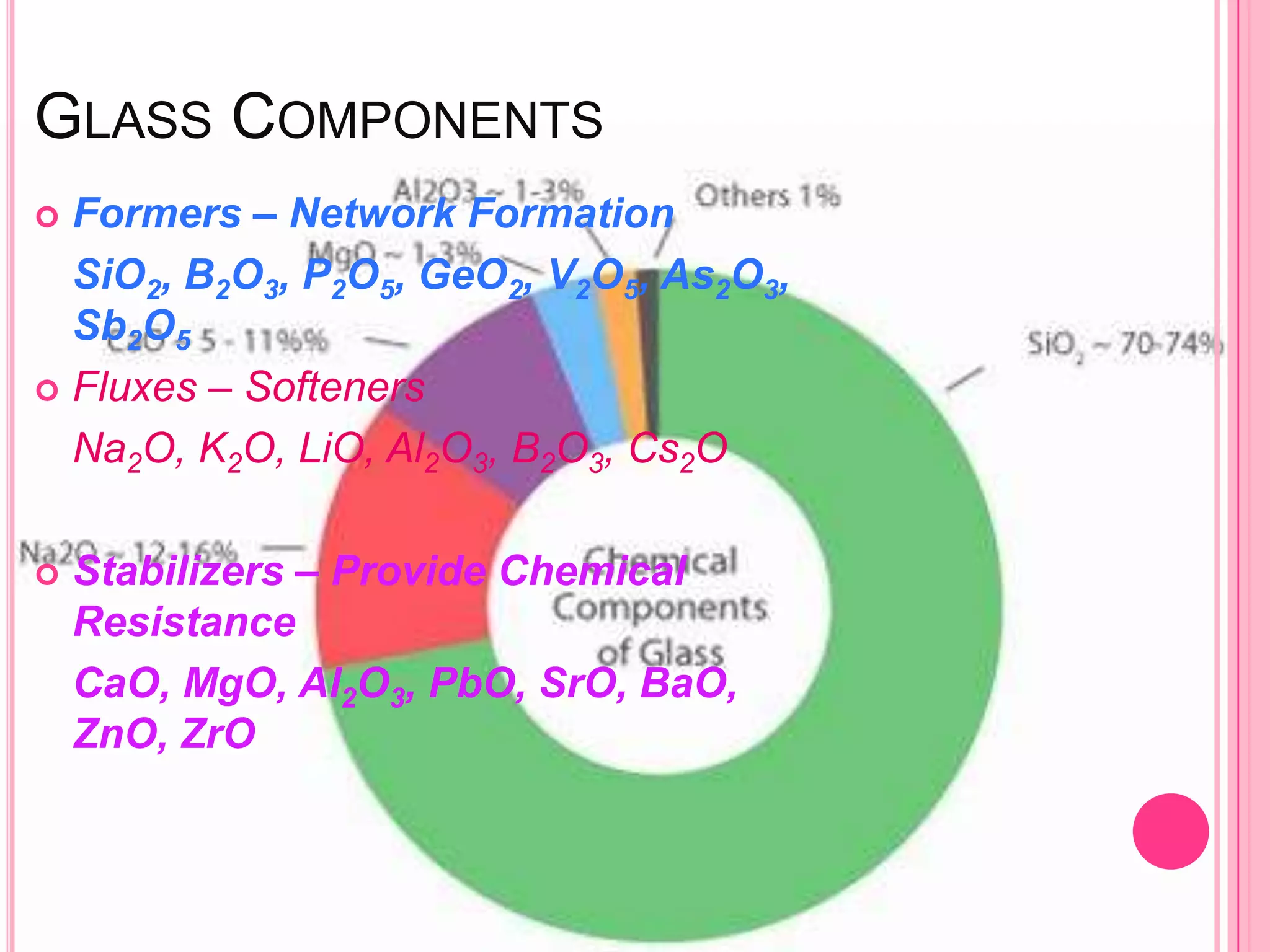
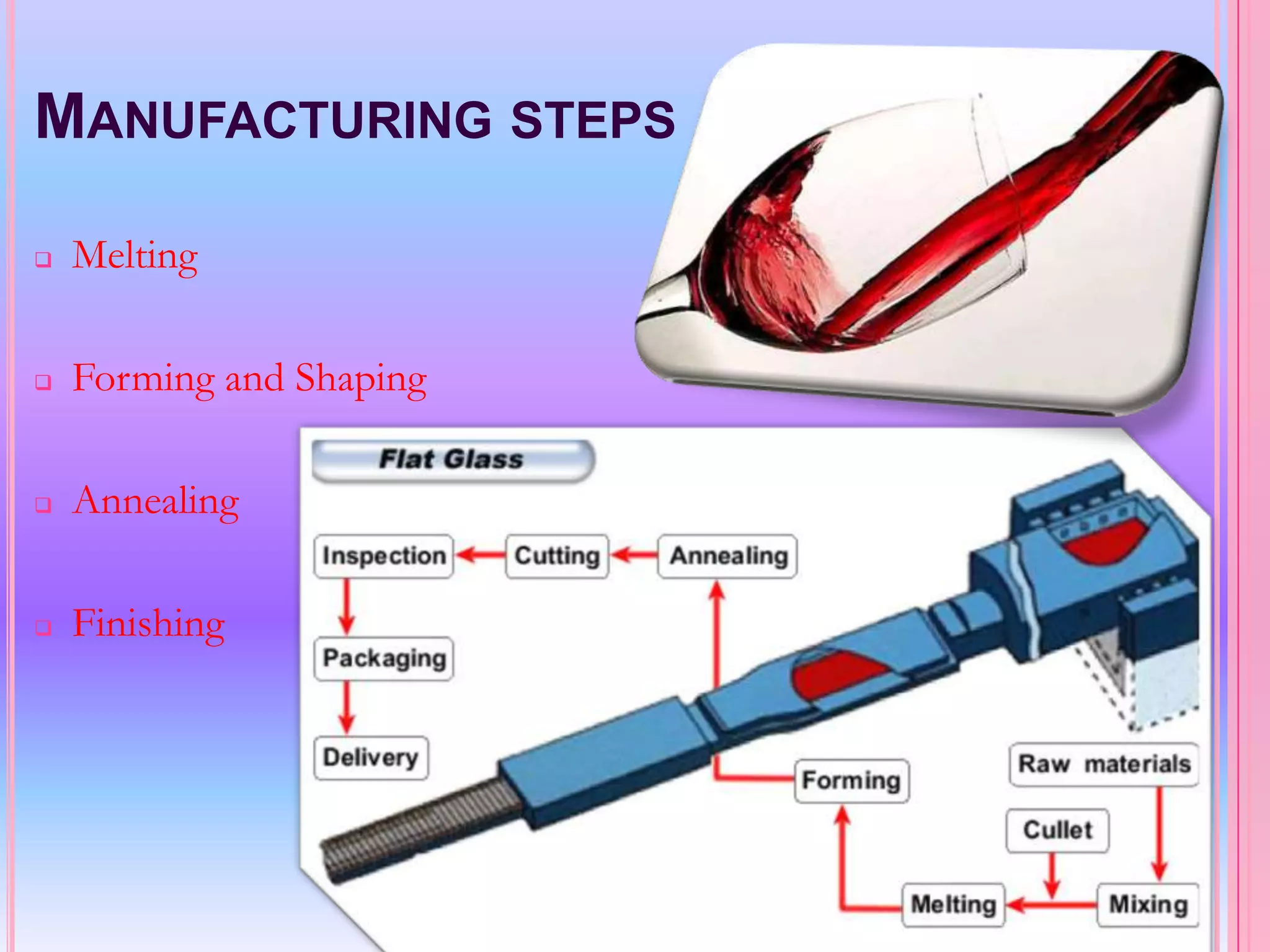
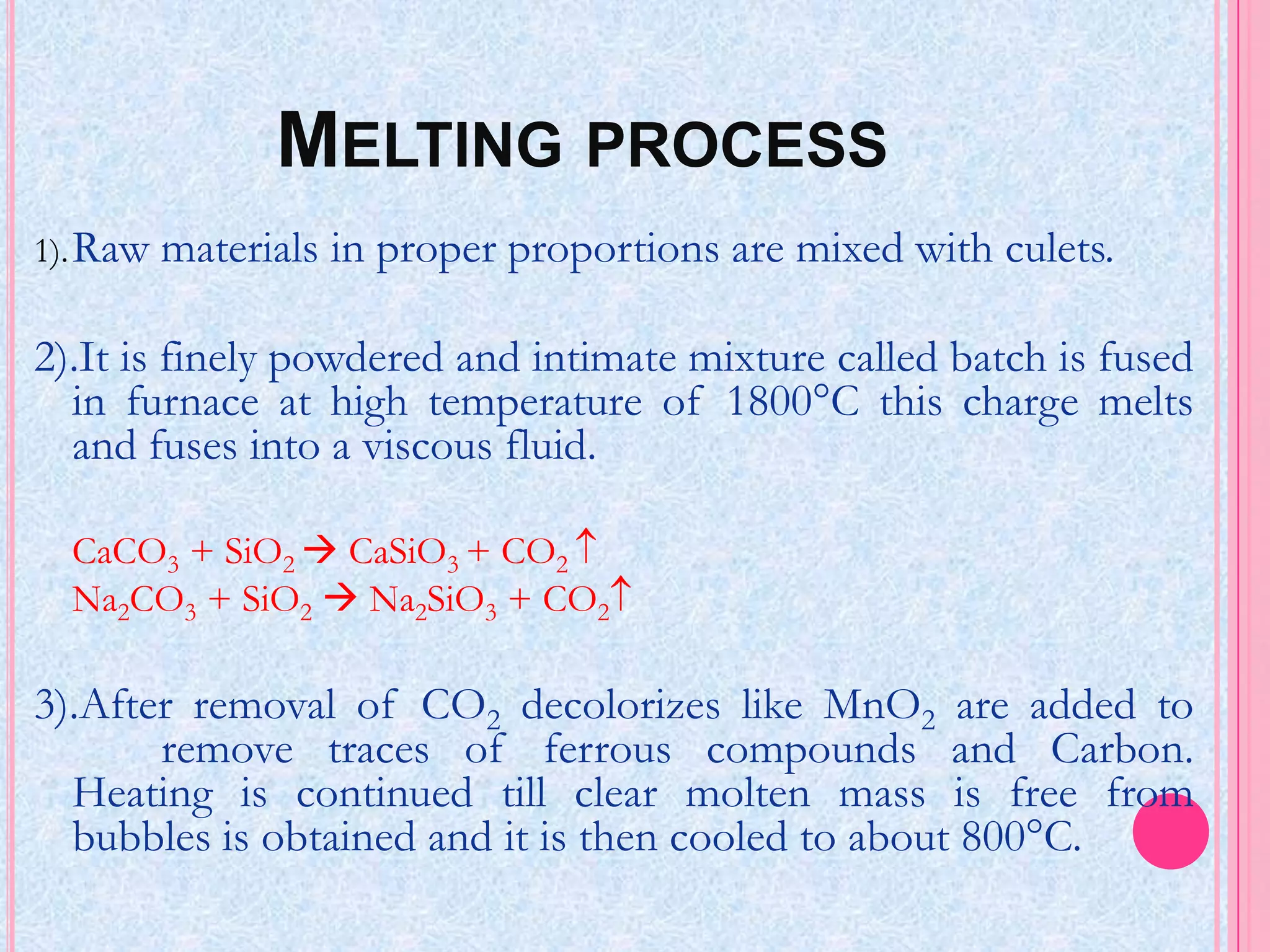
Glass is an amorphous, brittle material created from a mixture of metallic silicates or borates, notable for its lack of a definite melting point and high compressive strength. The manufacturing process involves melting raw materials, forming and shaping, and finishing to produce various types of glass like soda-lime, potash-lime, and lead glass, each with specific properties and uses. Applications range from everyday items like bottles and windows to specialized glassware used in laboratories and for optical purposes.