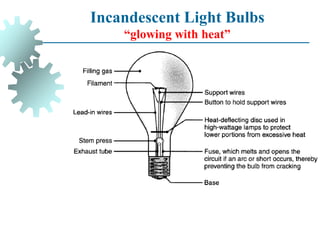
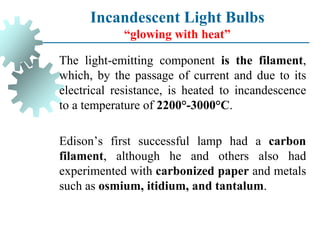
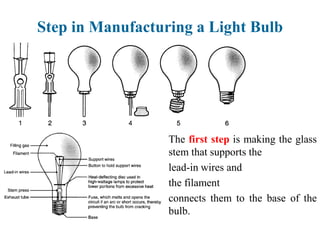
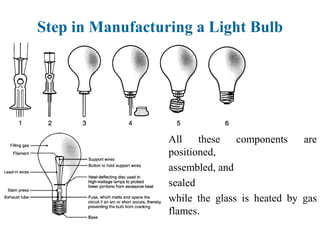
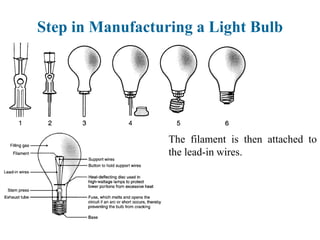
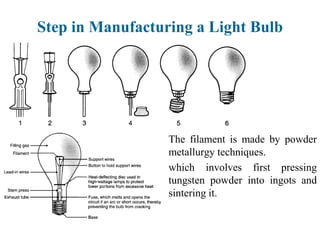
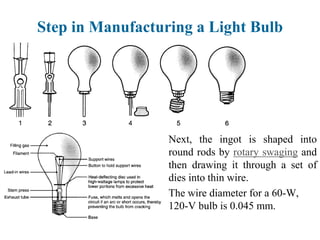
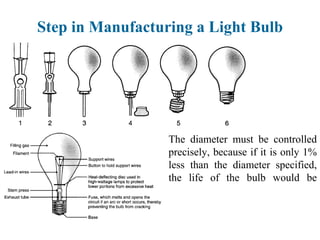
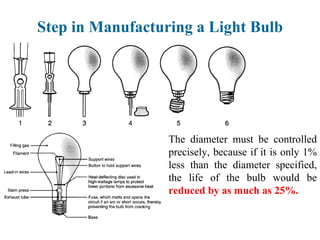
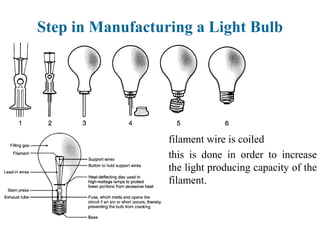
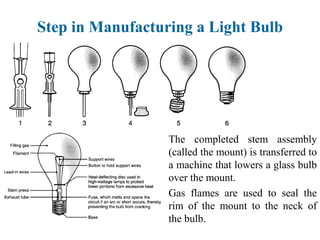
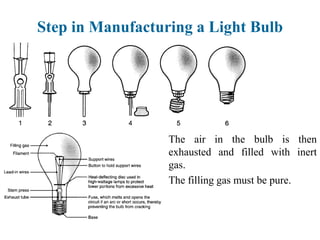
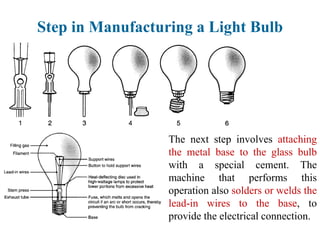
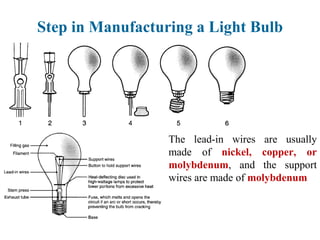
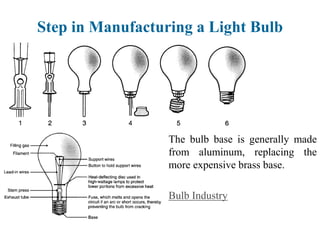
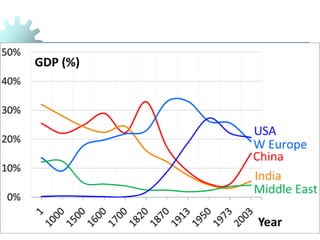
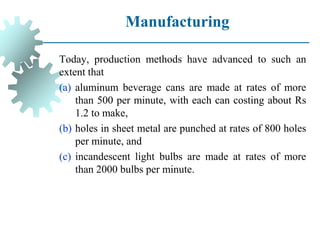
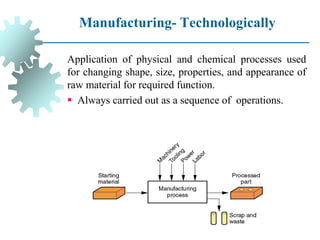
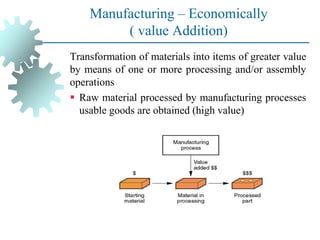
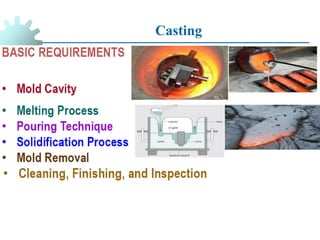
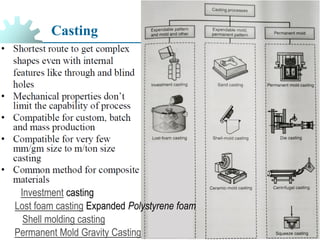
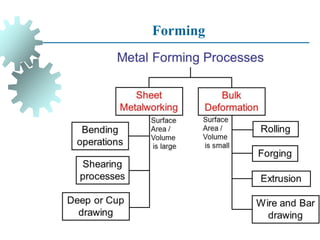
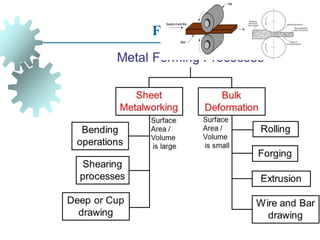
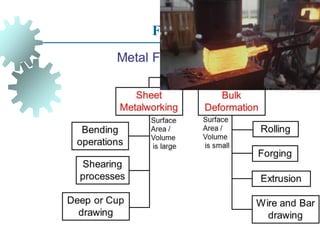
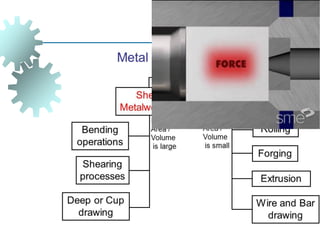
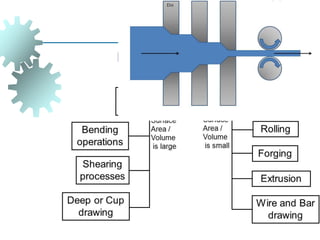
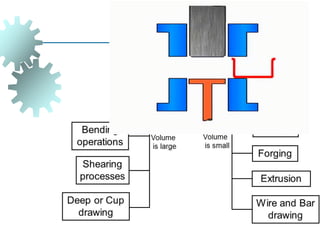
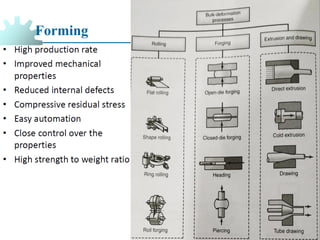
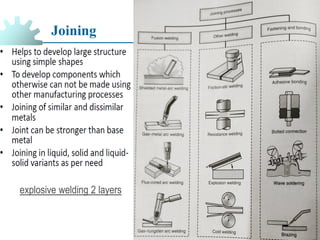
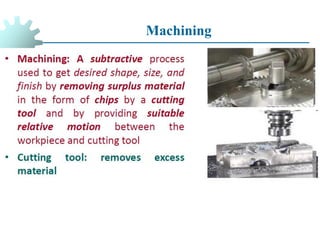
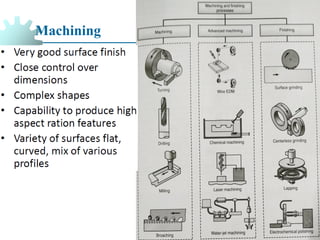
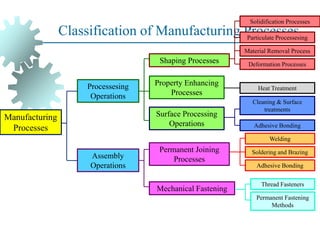
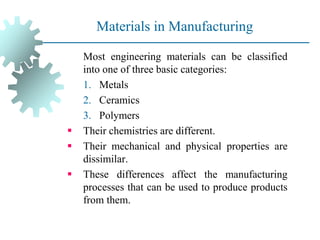
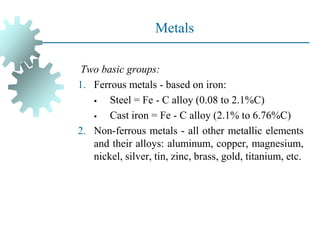
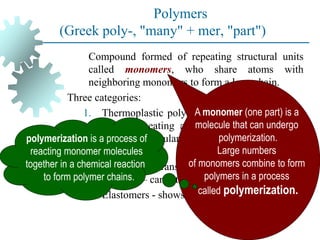
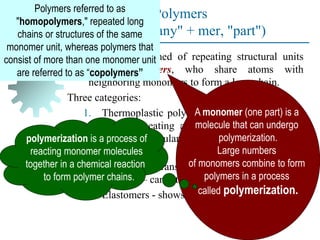
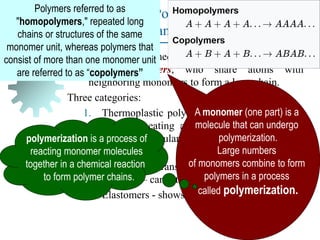
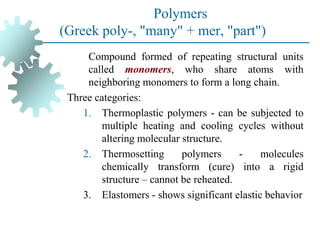
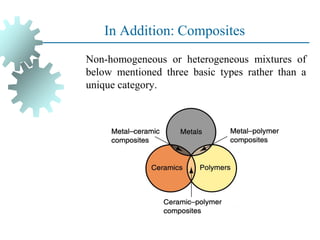
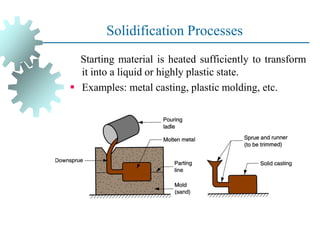
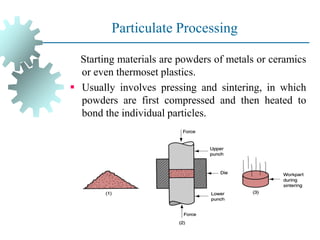
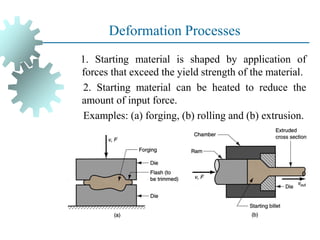
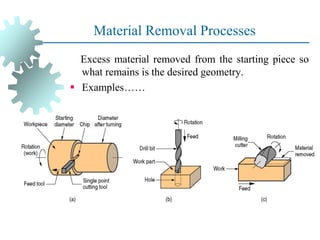
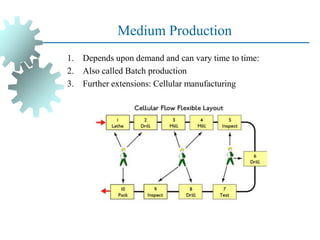
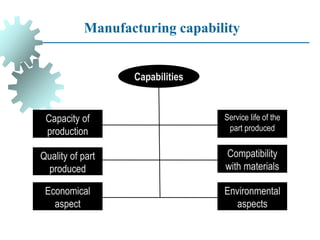
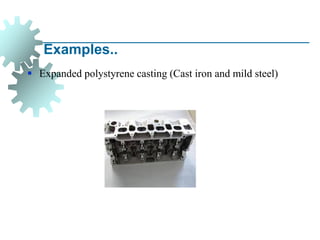
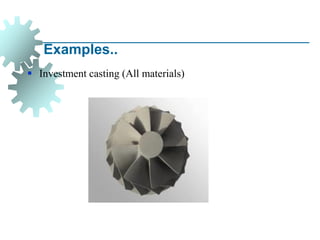
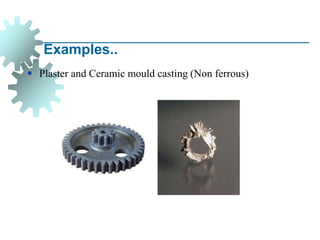
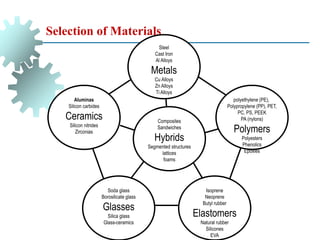
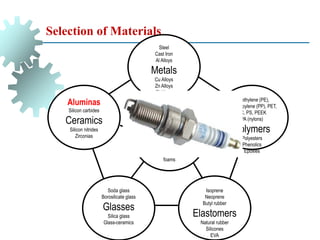
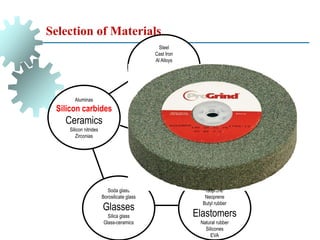
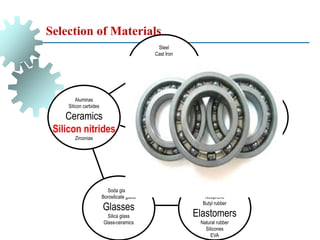
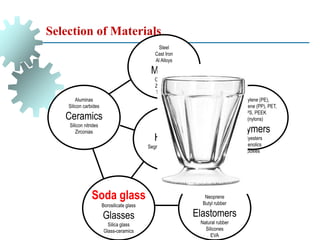
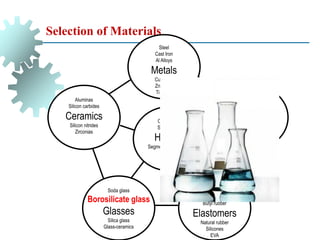
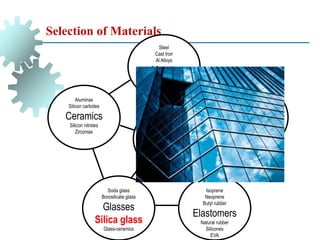
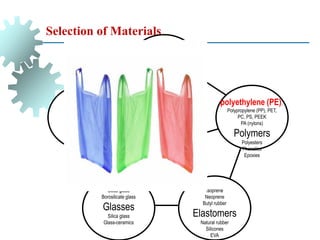
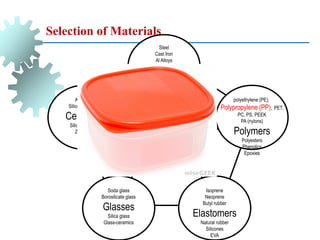
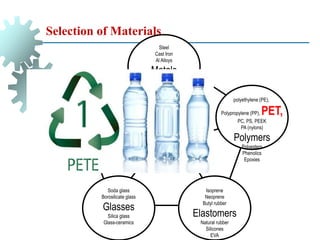
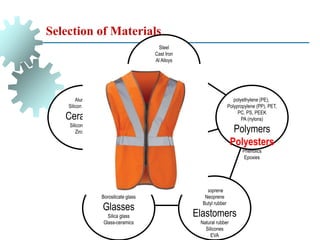
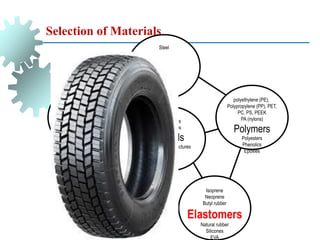
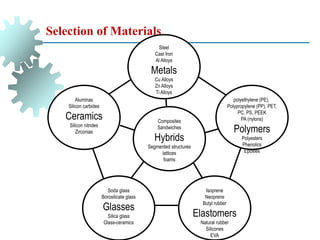
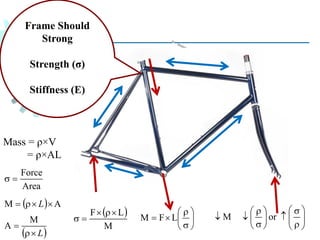
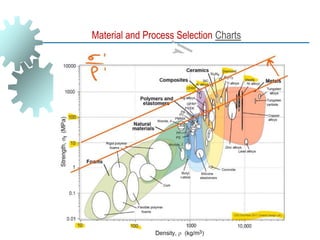
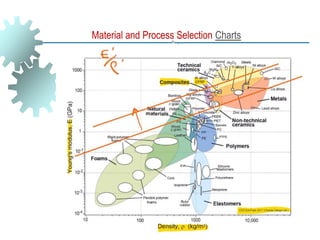
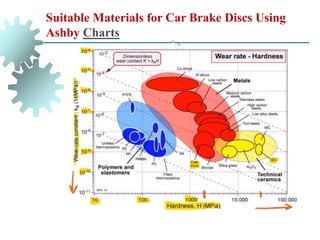
The document provides a comprehensive overview of manufacturing processes, emphasizing the importance of productivity and resource efficiency. It covers the history, materials, and techniques used in manufacturing incandescent light bulbs, as well as the categorization of materials into metals, ceramics, and polymers, along with various manufacturing processes. Additionally, it explores the principles of production types and capabilities, highlighting the significance of minimizing waste and optimizing material selection.