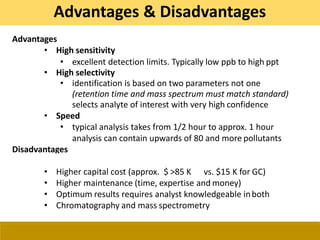
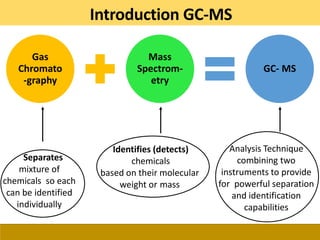
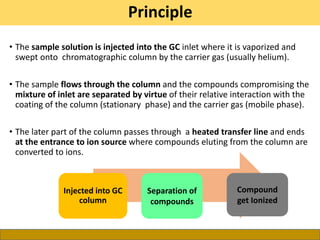
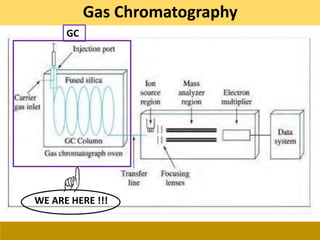
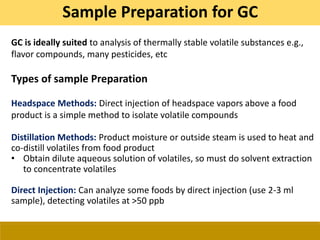
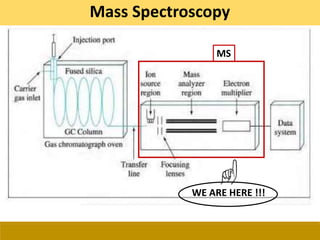
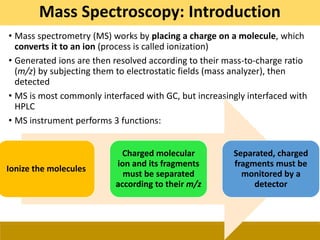
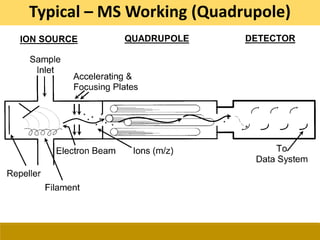
This document provides a comprehensive overview of gas chromatography-mass spectrometry (GC-MS), detailing its principles, working mechanisms, and applications in chemical analysis. It explains sample preparation techniques, types of gas supplies, column designs, and various interfacing methods between GC and MS. The document also discusses the mass spectrometry process, ionization methods, and the advantages and disadvantages of GC-MS analysis.

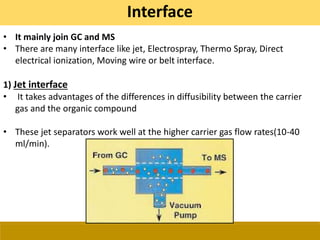
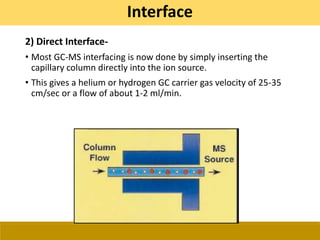
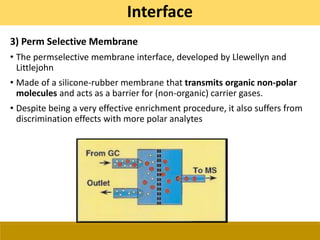
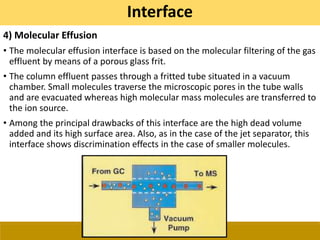
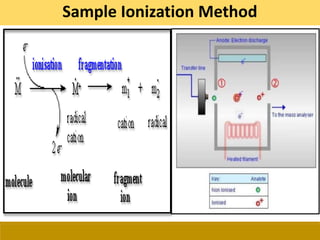
![Ionization
method
Typical
Analytes
Sample
Introduction
Mass
Range
Method
Highlights
Electron Impact (EI)
Relatively
small
volatile
GC or
liquid/solid
probe
to
1,000
Daltons
Hard
method
versatile
provides
structure
info
Chemical Ionization (CI)
Relatively
small
volatile
GC or
liquid/solid
probe
to
1,000
Daltons
Soft
method
molecular
ion peak
[M+H]+
Electrospray (ESI)
Peptides
Proteins
nonvolatile
Liquid
Chromatography or
syringe
to
200,000
Daltons
Soft
method
ions often
multiply
charged
Sample Ionization Method](https://image.slidesharecdn.com/gc-ms-210131160457/85/Gas-Chromatography-and-Mass-Spectroscopy-23-320.jpg)