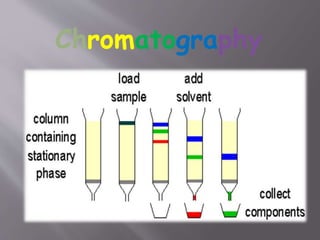
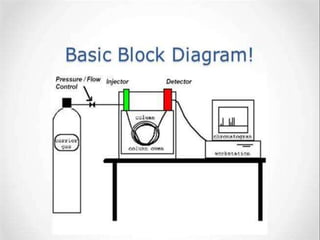
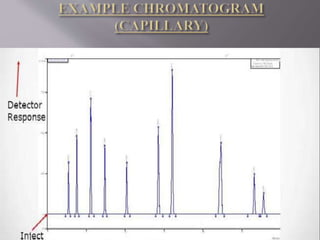
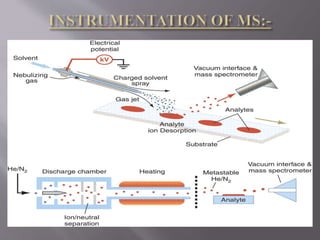
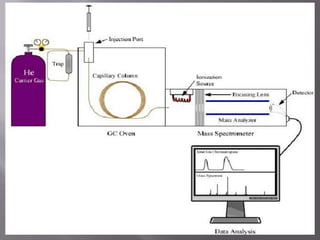
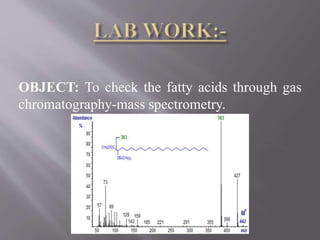
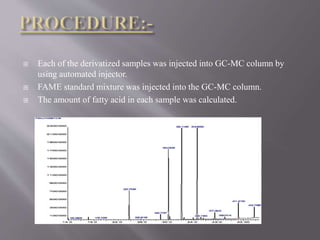
The document discusses gas chromatography-mass spectrometry (GC-MS), a hyphenated analytical technique that combines gas chromatography and mass spectrometry. GC-MS separates the components of a mixture and allows each component to be characterized individually. It has various applications including environmental monitoring, food and flavor analysis, and forensic and pharmaceutical analysis. The document provides details on the working principles, instrumentation, and uses of GC-MS.