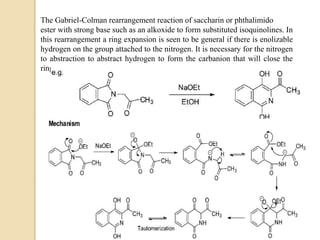
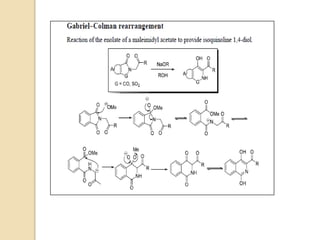
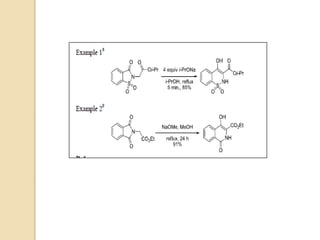
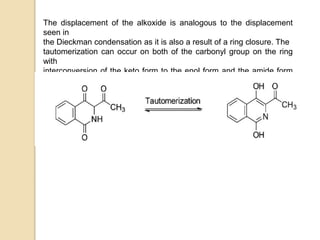
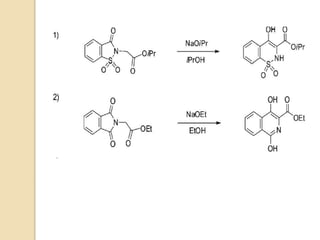
The Gabriel-Colman rearrangement reaction involves the rearrangement of saccharin or phthalimido ester with a strong base such as an alkoxide to form substituted isoquinolines. This is a ring expansion reaction that requires the nitrogen atom to abstract an enolizable hydrogen to form a carbanion that closes the ring. The displacement of the alkoxide is analogous to the Dieckman condensation, resulting in ring closure. The carbonyl groups on the ring can tautomerize between keto and enol forms, and the amide can tautomerize to an imidic acid form.