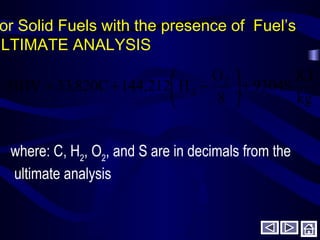
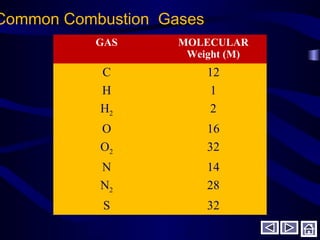
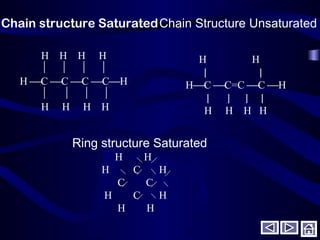
This document discusses fuels and combustion. It defines fuels and combustion, lists common types of fuels including solid, liquid, gaseous and nuclear fuels. It explains the concepts of complete and incomplete combustion and the oxidation of carbon, hydrogen and sulfur during combustion. It discusses air composition, theoretical air requirements, and combustion of hydrocarbon fuels with both theoretical and excess air. It also covers combustion of solid fuels and their analysis, as well as heating values and properties of fuels and lubricants.






















![fuelkg
airkg
18f32e28d32c2b12a
3.76(28)g32g
F
A
t +++++
+
=
Theoretical air-fuel ratio:
Actual air-fuel ratio:
[ ]
fuelkg
airkg
18f32e28d32c2b12a
3.76(28)g32gx)(1
aF
A
+++++
++
=
](https://image.slidesharecdn.com/fuelsandcombustion2013-130911073639-phpapp01/85/Fuels-and-combustion-2013-27-320.jpg)